Methods for measuring myeloperoxidase activity toward assessing inhibitor efficacy in living systems
- PMID: 26884610
- PMCID: PMC6608033
- DOI: 10.1189/jlb.3RU0615-256R
Methods for measuring myeloperoxidase activity toward assessing inhibitor efficacy in living systems
Abstract
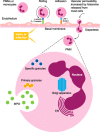
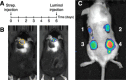
Myeloperoxidase aids in clearance of microbes by generation of peroxidase-mediated oxidants that kill leukocyte-engulfed pathogens. In this review, we will examine 1) strategies for in vitro evaluation of myeloperoxidase function and its inhibition, 2) ways to monitor generation of certain oxidant species during inflammation, and 3) how these methods can be used to approximate the total polymorphonuclear neutrophil chemotaxis following insult. Several optical imaging probes are designed to target reactive oxygen and nitrogen species during polymorphonuclear neutrophil inflammatory burst following injury. Here, we review the following 1) the broad effect of myeloperoxidase on normal physiology, 2) the difference between myeloperoxidase and other peroxidases, 3) the current optical probes available for use as surrogates for direct measures of myeloperoxidase-derived oxidants, and 4) the range of preclinical options for imaging myeloperoxidase accumulation at sites of inflammation in mice. We also stress the advantages and drawbacks of each of these methods, the pharmacokinetic considerations that may limit probe use to strictly cell cultures for some reactive oxygen and nitrogen species, rather than in vivo utility as indicators of myeloperoxidase function. Taken together, our review should shed light on the fundamental rational behind these techniques for measuring myeloperoxidase activity and polymorphonuclear neutrophil response after injury toward developing safe myeloperoxidase inhibitors as potential therapy for chronic obstructive pulmonary disease and rheumatoid arthritis.
Keywords: bioluminescence; neutrophil chemotaxis; noninvasive imaging; reactive nitrogen species; reactive oxygen species.
© Society for Leukocyte Biology.
Figures


References
-
- Auffray, C. , Fogg, D. , Garfa, M. , Elain, G. , Join‐Lambert, O. , Kayal, S. , Sarnacki, S. , Cumano, A. , Lauvau, G. , Geissmann, F. (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670. - PubMed
-
- Theilgaard‐Mönch, K. , Porse, B. T. , Borregaard, N. (2006) Systems biology of neutrophil differentiation and immune response. Curr. Opin. Immunol. 18, 54–60. - PubMed
-
- Diacovo, T. G. , Roth, S. J. , Buccola, J. M. , Bainton, D. F. , Springer, T. A. (1996) Neutrophil rolling, arrest, and transmigration across activated, surface‐adherent platelets via sequential action of P‐selectin and the beta 2‐integrin CD11b/CD18. Blood 88, 146–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

