Caspase-3/7-mediated Cleavage of β2-spectrin is Required for Acetaminophen-induced Liver Damage
- PMID: 26884715
- PMCID: PMC4737674
- DOI: 10.7150/ijbs.13420
Caspase-3/7-mediated Cleavage of β2-spectrin is Required for Acetaminophen-induced Liver Damage
Abstract
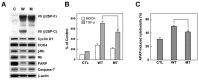
The ubiquitously expressed β2-spectrin (β2SP, SPTBN1) is the most common non-erythrocytic member of the β-spectrin gene family. Loss of β2-spectrin leads to defects in liver development, and its haploinsufficiency spontaneously leads to chronic liver disease and the eventual development of hepatocellular cancer. However, the specific role of β2-spectrin in liver homeostasis remains to be elucidated. Here, we reported that β2-spectrin was cleaved by caspase-3/7 upon treatment with acetaminophen which is the main cause of acute liver injury. Blockage of β2-spectrin cleavage robustly attenuated β2-spectrin-specific functions, including regulation of the cell cycle, apoptosis, and transcription. Cleaved fragments of β2-spectrin were physiologically active, and the N- and C-terminal fragments retained discrete interaction partners and activity in transcriptional regulation and apoptosis, respectively. Cleavage of β2-spectrin facilitated the redistribution of the resulting fragments under conditions of liver damage induced by acetaminophen. In contrast, downregulation of β2-spectrin led to resistance to acetaminophen-induced cytotoxicity, and its insufficiency in the liver promoted suppression of acetaminophen-induced liver damage and enhancement of liver regeneration.
Conclusions: β2-Spectrin, a TGF-β mediator and signaling molecule, is cleaved and activated by caspase-3/7, consequently enhancing apoptosis and transcriptional control to determine cell fate upon liver damage. These findings have extended our knowledge on the spectrum of β2-spectrin functions from a scaffolding protein to a target and transmitter of TGF-β in liver damage.
Keywords: TGF-β; acetaminophen; caspase-3/7; liver damage; β2-spectrin.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Viel A, Branton D. Spectrin: on the path from structure to function. Curr Opin Cell Biol. 1996;8:49–55. - PubMed
-
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990;70:1029–65. - PubMed
-
- Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–7. - PubMed
-
- Mishra B, Tang Y, Katuri V, Fleury T, Said AH, Rashid A. et al. Loss of cooperative function of transforming growth factor-beta signaling proteins, smad3 with embryonic liver fodrin, a beta-spectrin, in primary biliary cirrhosis. Liver Int. 2004;24:637–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

