Protective effects of ginsenoside Rg2 against H2O2-induced injury and apoptosis in H9c2 cells
- PMID: 26884906
- PMCID: PMC4723751
Protective effects of ginsenoside Rg2 against H2O2-induced injury and apoptosis in H9c2 cells
Abstract
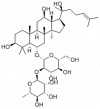
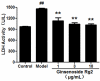
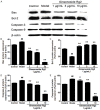
Ginsenoside Rg2 is one of the major active components of ginseng and has many biological activities. This study aimed to investigate the protective effects of ginsenoside Rg2 against H2O2-induced injury and apoptosis in H9c2 cells. The results showed that pretreatment with ginsenoside Rg2 not only increased cell viability, but also decreased lactate dehydrogenase (LDH) release. Ginsenoside Rg2 inhibited the decrease of SOD, GSH-PX activities and the increase of MDA content induced by H2O2. Meanwhile, the levels of ROS generation and cardiomyocyte apoptosis in ginsenoside Rg2 group significantly reduced when compared with the model group. Western blot analyses demonstrated that ginsenoside Rg2 up-regulate level of Bcl-2 expression and down-regulate levels of Bax, Caspase-3, -9 expression. These findings indicated that ginsenoside Rg2 could protect H9c2 cells against H2O2-induced injury through its actions of anti-oxidant and anti-apoptosis.
Keywords: Ginsenoside Rg2; apoptosis; hydrogen peroxide; oxidative stress.
Figures





Similar articles
-
Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Aβ25-35 in rats.J Ethnopharmacol. 2021 Feb 10;266:113466. doi: 10.1016/j.jep.2020.113466. Epub 2020 Oct 10. J Ethnopharmacol. 2021. PMID: 33049344
-
Ginsenoside Rg2 protects PC12 cells against β-amyloid25-35-induced apoptosis via the phosphoinositide 3-kinase/Akt pathway.Chem Biol Interact. 2017 Sep 25;275:152-161. doi: 10.1016/j.cbi.2017.07.021. Epub 2017 Jul 26. Chem Biol Interact. 2017. PMID: 28756148
-
20(S)-Ginsenoside Rg2 attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation: role of SIRT1.RSC Adv. 2018 Jul 2;8(42):23947-23962. doi: 10.1039/c8ra02316f. eCollection 2018 Jun 27. RSC Adv. 2018. PMID: 35540288 Free PMC article.
-
Schisandra chinensis bee pollen's chemical profiles and protective effect against H2O2-induced apoptosis in H9c2 cardiomyocytes.BMC Complement Med Ther. 2020 Sep 10;20(1):274. doi: 10.1186/s12906-020-03069-1. BMC Complement Med Ther. 2020. PMID: 32912207 Free PMC article.
-
Protective Effect of Rosamultin against H2O2-Induced Oxidative Stress and Apoptosis in H9c2 Cardiomyocytes.Oxid Med Cell Longev. 2018 Jul 16;2018:8415610. doi: 10.1155/2018/8415610. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30116494 Free PMC article.
Cited by
-
Upregulation of autophagy by Ginsenoside Rg2 in MCF-7 cells.Anim Cells Syst (Seoul). 2018 Nov 19;22(6):382-389. doi: 10.1080/19768354.2018.1545696. eCollection 2018. Anim Cells Syst (Seoul). 2018. PMID: 30533260 Free PMC article.
-
Ginseng extract and ginsenosides improve neurological function and promote antioxidant effects in rats with spinal cord injury: A meta-analysis and systematic review.J Ginseng Res. 2022 Jan;46(1):11-22. doi: 10.1016/j.jgr.2021.05.009. Epub 2021 Jun 18. J Ginseng Res. 2022. PMID: 35058723 Free PMC article. Review.
-
Panax ginseng and Panax quinquefolius: From pharmacology to toxicology.Food Chem Toxicol. 2017 Sep;107(Pt A):362-372. doi: 10.1016/j.fct.2017.07.019. Epub 2017 Jul 8. Food Chem Toxicol. 2017. PMID: 28698154 Free PMC article. Review.
-
Antiarrhythmic effects of ginsenoside Rg2 on calcium chloride-induced arrhythmias without oral toxicity.J Ginseng Res. 2020 Sep;44(5):717-724. doi: 10.1016/j.jgr.2019.06.005. Epub 2019 Jun 22. J Ginseng Res. 2020. PMID: 32913401 Free PMC article.
-
Inhibitory Effect of Lotus Leaf-Enriched Flavonoid Extract on the Growth of HT-29 Colon Cancer Cells through the Expression of PI3K-Related Molecules.Biomed Res Int. 2022 May 9;2022:6770135. doi: 10.1155/2022/6770135. eCollection 2022. Biomed Res Int. 2022. PMID: 35586809 Free PMC article.
References
-
- Jiang H, Ge J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart. 2009;95:1727–1731. - PubMed
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. - PMC - PubMed
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. - PubMed
-
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. - PubMed
-
- Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–418. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
