An In Vitro Comparison of PMMA and Calcium Sulfate as Carriers for the Local Delivery of Gallium(III) Nitrate to Staphylococcal Infected Surgical Sites
- PMID: 26885514
- PMCID: PMC4739006
- DOI: 10.1155/2016/7078989
An In Vitro Comparison of PMMA and Calcium Sulfate as Carriers for the Local Delivery of Gallium(III) Nitrate to Staphylococcal Infected Surgical Sites
Abstract
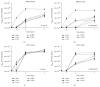
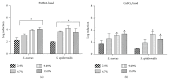
Antibiotic-loaded bone cements, including poly(methyl methacrylate) (PMMA) and calcium sulfate (CaSO4), are often used for treatment of orthopaedic infections involving Staphylococcus spp., although the effectiveness of this treatment modality may be limited due to the emergence of antimicrobial resistance and/or the development of biofilms within surgical sites. Gallium(III) is an iron analog capable of inhibiting essential iron-dependent pathways, exerting broad antimicrobial activity against multiple microorganisms, including Staphylococcus spp. Herein, we evaluated PMMA and CaSO4 as carriers for delivery of gallium(III) nitrate (Ga(NO3)3) to infected surgical sites by assessing the release kinetics subsequent to incorporation and antimicrobial activity against S. aureus and S. epidermidis. PMMA and to a lesser extent CaSO4 were observed to be compatible as carriers for Ga(NO3)3, eluting concentrations with antimicrobial activity against planktonic bacteria, inhibiting bacterial growth, and preventing bacterial colonization of beads, and effective against established bacterial biofilms of S. aureus and S. epidermidis. Collectively, our in vitro results indicate that PMMA is a more suitable carrier compared to CaSO4 for delivery of Ga(NO3)3; moreover they provide evidence for the potential use of Ga(NO3)3 with PMMA as a strategy for the prevention and/or treatment for orthopaedic infections.
Figures



Similar articles
-
Effects of loading concentration, blood and synovial fluid on antibiotic release and anti-biofilm activity of bone cement beads.J Control Release. 2017 Feb 28;248:24-32. doi: 10.1016/j.jconrel.2017.01.005. Epub 2017 Jan 10. J Control Release. 2017. PMID: 28087408
-
The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus.Biomaterials. 2012 Jan;33(2):365-77. doi: 10.1016/j.biomaterials.2011.09.084. Epub 2011 Oct 20. Biomaterials. 2012. PMID: 22014946
-
Rifamycin Derivatives Are Effective Against Staphylococcal Biofilms In Vitro and Elutable From PMMA.Clin Orthop Relat Res. 2015 Sep;473(9):2874-84. doi: 10.1007/s11999-015-4300-3. Clin Orthop Relat Res. 2015. PMID: 25896136 Free PMC article.
-
The use of antimicrobial-impregnated PMMA to manage periprosthetic infections: controversial issues and the latest developments.Int J Artif Organs. 2012 Oct;35(10):832-9. doi: 10.5301/ijao.5000163. Int J Artif Organs. 2012. PMID: 23138709 Review.
-
Daptomycin in bone and joint infections: a review of the literature.Arch Orthop Trauma Surg. 2009 Nov;129(11):1495-504. doi: 10.1007/s00402-008-0772-x. Epub 2008 Nov 7. Arch Orthop Trauma Surg. 2009. PMID: 18989686 Free PMC article. Review.
Cited by
-
Nonconventional Therapeutics against Staphylococcus aureus.Microbiol Spectr. 2018 Nov;6(6):10.1128/microbiolspec.gpp3-0047-2018. doi: 10.1128/microbiolspec.GPP3-0047-2018. Microbiol Spectr. 2018. PMID: 30547858 Free PMC article.
-
Adjustment of Micro- and Macroporosity of ß-TCP Scaffolds Using Solid-Stabilized Foams as Bone Replacement.Bioengineering (Basel). 2023 Feb 15;10(2):256. doi: 10.3390/bioengineering10020256. Bioengineering (Basel). 2023. PMID: 36829750 Free PMC article.
-
Colonization and Infection of Indwelling Medical Devices by Staphylococcus aureus with an Emphasis on Orthopedic Implants.Int J Mol Sci. 2022 May 25;23(11):5958. doi: 10.3390/ijms23115958. Int J Mol Sci. 2022. PMID: 35682632 Free PMC article. Review.
-
Connecting iron acquisition and biofilm formation in the ESKAPE pathogens as a strategy for combatting antibiotic resistance.Medchemcomm. 2019 Mar 21;10(4):505-512. doi: 10.1039/c9md00032a. eCollection 2019 Apr 1. Medchemcomm. 2019. PMID: 31057729 Free PMC article. Review.
-
Gallium: a decisive "Trojan Horse" against microorganisms.Antonie Van Leeuwenhoek. 2024 Sep 13;118(1):3. doi: 10.1007/s10482-024-02015-2. Antonie Van Leeuwenhoek. 2024. PMID: 39269546 Review.
References
-
- Ostermann P. A. W., Seligson D., Henry S. L. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. Journal of Bone and Joint Surgery Series: B. 1995;77(1):93–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

