Oral Polio Vaccination and Hospital Admissions With Non-Polio Infections in Denmark: Nationwide Retrospective Cohort Study
- PMID: 26885538
- PMCID: PMC4751340
- DOI: 10.1093/ofid/ofv204
Oral Polio Vaccination and Hospital Admissions With Non-Polio Infections in Denmark: Nationwide Retrospective Cohort Study
Abstract
Background. Live vaccines may have nonspecific beneficial effects on morbidity and mortality. This study examines whether children who had the live-attenuated oral polio vaccine (OPV) as the most recent vaccine had a different rate of admissions for infectious diseases than children with inactivated diphtheria-tetanus-pertussis-polio-Haemophilus influenzae type b vaccine (DTaP-IPV-Hib) or live measles-mumps-rubella vaccine (MMR) as their most recent vaccine. Methods. A nationwide, register-based, retrospective cohort study of 137 403 Danish children born 1997-1999, who had received 3 doses of DTaP-IPV-Hib, were observed from 24 months (first OPV dose) to 36 months of age. Results. Oral polio vaccine was associated with a lower rate of admissions with any type of non-polio infection compared with DTaP-IPV-Hib as most recent vaccine (adjusted incidence rate ratio [IRR], 0.85; 95% confidence interval [CI], .77-.95). The association was separately significant for admissions with lower respiratory infections (adjusted IRR, 0.73; 95% CI, .61-.87). The admission rates did not differ for OPV versus MMR. Conclusions. Like MMR, OPV was associated with fewer admissions for lower respiratory infections than having DTaP-IPV-Hib as the most recent vaccination. Because OPV is now being phased-out globally, further studies of the potential beneficial nonspecific effects of OPV are warranted.
Keywords: heterologous immunity; immunization; nonspecific effects; nontargeted effects; oral polio vaccination.
Figures
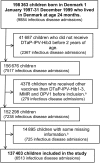

References
-
- Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 – conclusions and recommendations. Wkly Epidemiol Rec 2014; 89:221–36. - PubMed
-
- Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. Available at: http://www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiol... Accessed 23 October 2014.
-
- Contreras G. Effect of the administration of oral poliovirus vaccine on infantile diarrhoea mortality. Vaccine 1989; 7:211–2. - PubMed
-
- Contreras G. Sabin's vaccine used for nonspecific prevention of infant diarrhea of viral etiology. Bull Pan Am Health Organ 1974; 8:123–32. - PubMed
-
- Aaby P, Rodrigues A, Biai S et al. . Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine 2004; 22:3014–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

