Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats
- PMID: 26885754
- PMCID: PMC4890982
- DOI: 10.18632/oncotarget.7381
Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats
Abstract
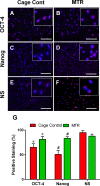
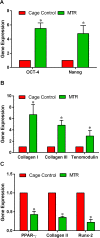
The effect of exercise on wound healing in aging tendon was tested using a rat moderate treadmill running (MTR) model. The rats were divided into an MTR group that ran on a treadmill for 4 weeks and a control group that remained in cages. After MTR, a window defect was created in the patellar tendons of all rats and wound healing was analyzed. We found that MTR accelerated wound healing by promoting quicker closure of wounds, improving the organization of collagen fibers, and decreasing senescent cells in the wounded tendons when compared to the cage control. MTR also lowered vascularization, increased the numbers of tendon stem/progenitor cells (TSCs) and TSC proliferation than the control. Besides, MTR significantly increased the expression of stem cell markers, OCT-4 and Nanog, and tenocyte genes, Collagen I, Collagen III and tenomodulin, and down-regulated PPAR-γ, Collagen II and Runx-2 (non-tenocyte genes). These findings indicated that moderate exercise enhances healing of injuries in aging tendons through TSC based mechanisms, through which exercise regulates beneficial effects in tendons. This study reveals that appropriate exercise may be used in clinics to enhance tendon healing in aging patients.
Keywords: Gerotarget; aging rat; proliferation; tendon stem cell; treadmill running; wound healing.
Conflict of interest statement
There is no conflict of interest.
Figures







Similar articles
-
Moderate Exercise Mitigates the Detrimental Effects of Aging on Tendon Stem Cells.PLoS One. 2015 Jun 18;10(6):e0130454. doi: 10.1371/journal.pone.0130454. eCollection 2015. PLoS One. 2015. PMID: 26086850 Free PMC article.
-
Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen.J Orthop Res. 2010 Sep;28(9):1178-83. doi: 10.1002/jor.21123. J Orthop Res. 2010. PMID: 20225313
-
Moderate and intensive mechanical loading differentially modulate the phenotype of tendon stem/progenitor cells in vivo.PLoS One. 2020 Dec 29;15(12):e0242640. doi: 10.1371/journal.pone.0242640. eCollection 2020. PLoS One. 2020. PMID: 33373386 Free PMC article.
-
Mechanobiology of young and aging tendons: In vivo studies with treadmill running.J Orthop Res. 2018 Feb;36(2):557-565. doi: 10.1002/jor.23761. Epub 2017 Nov 22. J Orthop Res. 2018. PMID: 28976604 Free PMC article. Review.
-
Basic mechanisms of tendon fatigue damage.J Shoulder Elbow Surg. 2012 Feb;21(2):158-63. doi: 10.1016/j.jse.2011.11.014. J Shoulder Elbow Surg. 2012. PMID: 22244058 Free PMC article. Review.
Cited by
-
Tendon stem/progenitor cell ageing: Modulation and rejuvenation.World J Stem Cells. 2019 Sep 26;11(9):677-692. doi: 10.4252/wjsc.v11.i9.677. World J Stem Cells. 2019. PMID: 31616543 Free PMC article. Review.
-
Biology of Tendon Stem Cells and Tendon in Aging.Front Genet. 2020 Jan 16;10:1338. doi: 10.3389/fgene.2019.01338. eCollection 2019. Front Genet. 2020. PMID: 32010194 Free PMC article. Review.
-
AQP1 modulates tendon stem/progenitor cells senescence during tendon aging.Cell Death Dis. 2020 Mar 18;11(3):193. doi: 10.1038/s41419-020-2386-3. Cell Death Dis. 2020. PMID: 32188840 Free PMC article.
-
Use of adult mesenchymal stromal cells in tissue repair: impact of physical exercise.Am J Physiol Cell Physiol. 2019 Oct 1;317(4):C642-C654. doi: 10.1152/ajpcell.00530.2018. Epub 2019 Jun 26. Am J Physiol Cell Physiol. 2019. PMID: 31241985 Free PMC article. Review.
-
Is exercise a senolytic medicine? A systematic review.Aging Cell. 2021 Jan;20(1):e13294. doi: 10.1111/acel.13294. Epub 2020 Dec 30. Aging Cell. 2021. PMID: 33378138 Free PMC article.
References
-
- Klatte-Schulz F, Pauly S, Scheibel M, Greiner S, Gerhardt C, Schmidmaier G, Wildemann B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. European cells & materials. 2012;24:74–89. - PubMed
-
- Goodman SA, May SA, Heinegard D, Smith RK. Tenocyte response to cyclical strain and transforming growth factor beta is dependent upon age and site of origin. Biorheology. 2004;41:613–628. - PubMed
-
- Dudhia J, Scott CM, Draper ER, Heinegard D, Pitsillides AA, Smith RK. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging cell. 2007;6:547–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

