Glycogen Synthase Kinase 3 Inactivation Drives T-bet-Mediated Downregulation of Co-receptor PD-1 to Enhance CD8(+) Cytolytic T Cell Responses
- PMID: 26885856
- PMCID: PMC4760122
- DOI: 10.1016/j.immuni.2016.01.018
Glycogen Synthase Kinase 3 Inactivation Drives T-bet-Mediated Downregulation of Co-receptor PD-1 to Enhance CD8(+) Cytolytic T Cell Responses
Abstract
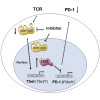
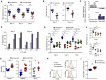
Despite the importance of the co-receptor PD-1 in T cell immunity, the upstream signaling pathway that regulates PD-1 expression has not been defined. Glycogen synthase kinase 3 (GSK-3, isoforms α and β) is a serine-threonine kinase implicated in cellular processes. Here, we identified GSK-3 as a key upstream kinase that regulated PD-1 expression in CD8(+) T cells. GSK-3 siRNA downregulation, or inhibition by small molecules, blocked PD-1 expression, resulting in increased CD8(+) cytotoxic T lymphocyte (CTL) function. Mechanistically, GSK-3 inactivation increased Tbx21 transcription, promoting enhanced T-bet expression and subsequent suppression of Pdcd1 (encodes PD-1) transcription in CD8(+) CTLs. Injection of GSK-3 inhibitors in mice increased in vivo CD8(+) OT-I CTL function and the clearance of murine gamma-herpesvirus 68 and lymphocytic choriomeningitis clone 13 and reversed T cell exhaustion. Our findings identify GSK-3 as a regulator of PD-1 expression and demonstrate the applicability of GSK-3 inhibitors in the modulation of PD-1 in immunotherapy.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H., Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. - PubMed
-
- Appleman L.J., Berezovskaya A., Grass I., Boussiotis V.A. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J. Immunol. 2000;164:144–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

