Avian Influenza A(H5N1) Virus in Egypt
- PMID: 26886164
- PMCID: PMC4766899
- DOI: 10.3201/eid2203.150593
Avian Influenza A(H5N1) Virus in Egypt
Abstract
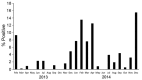
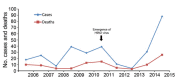
In Egypt, avian influenza A subtype H5N1 and H9N2 viruses are enzootic in poultry. The control plan devised by veterinary authorities in Egypt to prevent infections in poultry focused mainly on vaccination and ultimately failed. Recently, widespread H5N1 infections in poultry and a substantial increase in the number of human cases of H5N1 infection were observed. We summarize surveillance data from 2009 through 2014 and show that avian influenza viruses are established in poultry in Egypt and are continuously evolving genetically and antigenically. We also discuss the epidemiology of human infection with avian influenza in Egypt and describe how the true burden of disease is underestimated. We discuss the failures of relying on vaccinating poultry as the sole intervention tool. We conclude by highlighting the key components that need to be included in a new strategy to control avian influenza infections in poultry and humans in Egypt.
Keywords: Egypt; avian influenza; influenza; respiratory infections; subtype H5N1; surveillance; vaccination; vector-borne infections; viruses.
Figures




References
-
- World Health Organization. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2015 [cited 2015 Apr 15]. http://www.who.int/influenza/human_animal_interface/EN_GIP_20150303cumul...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

