Holographic Characterization of Protein Aggregates
- PMID: 26886303
- PMCID: PMC6292201
- DOI: 10.1016/j.xphs.2015.12.018
Holographic Characterization of Protein Aggregates
Abstract
We demonstrate how holographic video microscopy can be used to detect, count, and characterize individual micrometer-scale protein aggregates as they flow down a microfluidic channel in their native buffer. Holographic characterization directly measures the radius and refractive index of subvisible protein aggregates and offers insights into their morphologies. The measurement proceeds fast enough to build up population averages for time-resolved studies and lends itself to tracking trends in protein aggregation arising from changing environmental factors. Information on individual particle's refractive indexes can be used to differentiate protein aggregates from such contaminants as silicone droplets. These capabilities are demonstrated through measurements on samples of bovine pancreas insulin aggregated through centrifugation and of bovine serum albumin aggregated by complexation with a polyelectrolyte. Differentiation is demonstrated with samples that have been spiked with separately characterized silicone spheres. Holographic characterization measurements are compared with results obtained with microflow imaging and dynamic light scattering.
Keywords: biopharmaceuticals characterization; colloids; light scattering; microparticles; microscopy; particle size; protein aggregation.
Copyright © 2016 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
Figures



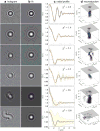
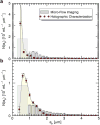
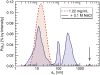


References
-
- Food and Drug Administration. Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products. Silver Spring, MD: U.S. Department of Health and Human Services; 2014.
-
- Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1:457–462. - PubMed
-
- Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289:1–30. - PubMed
-
- Singh SK, Afonina N, Awwad M, et al. An industry perspective on the monitoring of subvisible particles as a quality attribute for protein therapeutics. J Pharm Sci. 2010;99:3302–3321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

