Addition of Phenylboronic Acid to Malus domestica Pollen Tubes Alters Calcium Dynamics, Disrupts Actin Filaments and Affects Cell Wall Architecture
- PMID: 26886907
- PMCID: PMC4757038
- DOI: 10.1371/journal.pone.0149232
Addition of Phenylboronic Acid to Malus domestica Pollen Tubes Alters Calcium Dynamics, Disrupts Actin Filaments and Affects Cell Wall Architecture
Abstract
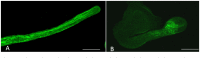
A key role of boron in plants is to cross-link the cell wall pectic polysaccharide rhamnogalacturonan-II (RG-II) through borate diester linkages. Phenylboronic acid (PBA) can form the same reversible ester bonds but cannot cross-link two molecules, so can be used as an antagonist to study the function of boron. This study aimed to evaluate the effect of PBA on apple (Malus domestica) pollen tube growth and the underlying regulatory mechanism. We observed that PBA caused an inhibition of pollen germination, tube growth and led to pollen tube morphological abnormalities. Fluorescent labeling, coupled with a scanning ion-selective electrode technique, revealed that PBA induced an increase in extracellular Ca2+ influx, thereby elevating the cytosolic Ca2+ concentration [Ca2+]c and disrupting the [Ca2+]c gradient, which is critical for pollen tube growth. Moreover the organization of actin filaments was severely perturbed by the PBA treatment. Immunolocalization studies and fluorescent labeling, together with Fourier-transform infrared analysis (FTIR) suggested that PBA caused an increase in the abundance of callose, de-esterified pectins and arabinogalactan proteins (AGPs) at the tip. However, it had no effect on the deposition of the wall polymers cellulose. These effects are similar to those of boron deficiency in roots and other organs, indicating that PBA can induce boron deficiency symptoms. The results provide new insights into the roles of boron in pollen tube development, which likely include regulating [Ca2+]c and the formation of the actin cytoskeleton, in addition to the synthesis and assembly of cell wall components.
Conflict of interest statement
Figures





Similar articles
-
Boron deficiency alters cytosolic Ca2+ concentration and affects the cell wall components of pollen tubes in Malus domestica.Plant Biol (Stuttg). 2019 Mar;21(2):343-351. doi: 10.1111/plb.12941. Epub 2018 Dec 19. Plant Biol (Stuttg). 2019. PMID: 30444945
-
Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes.New Phytol. 2009 Jun;182(4):851-862. doi: 10.1111/j.1469-8137.2009.02820.x. Epub 2009 Mar 30. New Phytol. 2009. PMID: 19646068
-
Boron Toxicity Causes Multiple Effects on Malus domestica Pollen Tube Growth.Front Plant Sci. 2016 Feb 26;7:208. doi: 10.3389/fpls.2016.00208. eCollection 2016. Front Plant Sci. 2016. PMID: 26955377 Free PMC article.
-
The function of actin-binding proteins in pollen tube growth.Protoplasma. 2007;230(3-4):171-82. doi: 10.1007/s00709-006-0231-x. Epub 2007 Apr 24. Protoplasma. 2007. PMID: 17458632 Review.
-
A review on the function of arabinogalactan-proteins during pollen grain development.Plant Reprod. 2025 Feb 6;38(1):8. doi: 10.1007/s00497-024-00515-9. Plant Reprod. 2025. PMID: 39912945 Free PMC article. Review.
Cited by
-
Evolution of Cell Wall Polymers in Tip-Growing Land Plant Gametophytes: Composition, Distribution, Functional Aspects and Their Remodeling.Front Plant Sci. 2019 Apr 18;10:441. doi: 10.3389/fpls.2019.00441. eCollection 2019. Front Plant Sci. 2019. PMID: 31057570 Free PMC article. Review.
-
From element to development: the power of the essential micronutrient boron to shape morphological processes in plants.J Exp Bot. 2020 Mar 12;71(5):1681-1693. doi: 10.1093/jxb/eraa042. J Exp Bot. 2020. PMID: 31985801 Free PMC article. Review.
-
Calreticulin is required for calcium homeostasis and proper pollen tube tip growth in Petunia.Planta. 2017 May;245(5):909-926. doi: 10.1007/s00425-017-2649-0. Epub 2017 Jan 11. Planta. 2017. PMID: 28078426 Free PMC article.
-
Let's shape again: the concerted molecular action that builds the pollen tube.Plant Reprod. 2022 Jun;35(2):77-103. doi: 10.1007/s00497-022-00437-4. Epub 2022 Jan 18. Plant Reprod. 2022. PMID: 35041045 Review.
-
Assessment of a 18F-Phenylboronic Acid Radiotracer for Imaging Boron in Maize.Int J Mol Sci. 2020 Feb 1;21(3):976. doi: 10.3390/ijms21030976. Int J Mol Sci. 2020. PMID: 32024118 Free PMC article.
References
-
- Camacho-Cristóbal JJ, Rexach J, González-Fontes A. Boron in Plants: deficiency and toxicity. J Int Plant Biol. 2008; 50: 1247–1255. - PubMed
-
- Martini F, Thellier M. Boron distribution in parenchyma cells of clover leaves. Plant Physiol Biochem. 1993; 31: 777–786.
-
- Ishii T, Matsunaga T. Pectic polysaccharide rhamnogalacturonan II is covalently linked to homogalacturonan. Phytochemisty 2001; 57: 969–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

