Multi-kinase inhibitors can associate with heat shock proteins through their NH2-termini by which they suppress chaperone function
- PMID: 26887051
- PMCID: PMC4914336
- DOI: 10.18632/oncotarget.7349
Multi-kinase inhibitors can associate with heat shock proteins through their NH2-termini by which they suppress chaperone function
Abstract
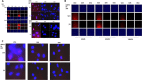
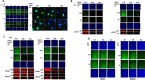
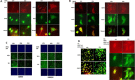
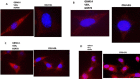
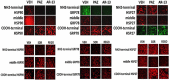
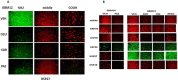
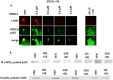
We performed proteomic studies using the GRP78 chaperone-inhibitor drug AR-12 (OSU-03012) as bait. Multiple additional chaperone and chaperone-associated proteins were shown to interact with AR-12, including: GRP75, HSP75, BAG2; HSP27; ULK-1; and thioredoxin. AR-12 down-regulated in situ immuno-fluorescence detection of ATP binding chaperones using antibodies directed against the NH2-termini of the proteins but only weakly reduced detection using antibodies directed against the central and COOH portions of the proteins. Traditional SDS-PAGE and western blotting assessment methods did not exhibit any alterations in chaperone detection. AR-12 altered the sub-cellular distribution of chaperone proteins, abolishing their punctate speckled patterning concomitant with changes in protein co-localization. AR-12 inhibited chaperone ATPase activity, which was enhanced by sildenafil; inhibited chaperone - chaperone and chaperone - client interactions; and docked in silico with the ATPase domains of HSP90 and of HSP70. AR-12 combined with sildenafil in a GRP78 plus HSP27 -dependent fashion to profoundly activate an eIF2α/ATF4/CHOP/Beclin1 pathway in parallel with inactivating mTOR and increasing ATG13 phosphorylation, collectively resulting in formation of punctate toxic autophagosomes. Over-expression of [GRP78 and HSP27] prevented: AR-12 -induced activation of ER stress signaling and maintained mTOR activity; AR-12 -mediated down-regulation of thioredoxin, MCL-1 and c-FLIP-s; and preserved tumor cell viability. Thus the inhibition of chaperone protein functions by AR-12 and by multi-kinase inhibitors very likely explains why these agents have anti-tumor effects in multiple genetically diverse tumor cell types.
Keywords: ATPase; OSU-03012; chaperones; pazopanib; sorafenib.
Conflict of interest statement
AZ and SP are Directors of Arno Therapeutics, the Corporation that owns the license for OSU-03012 (AR-12) from Ohio State University. CSC is the inventor of OSU-03012 and DW in the laboratory of CSC synthesized the biotin drug conjugate for our studies. All other authors have no conflicts of interest to report.
Figures







References
-
- Carón RW, Yacoub A, Li M, Zhu X, Mitchell C, Hong Y, Hawkins W, Sasazuki T, Shirasawa S, Kozikowski AP, Dennis PA, Hagan MP, Grant S, et al. Activated forms of H-RAS and K-RAS differentially regulate membrane association of PI3K, PDK-1, and AKT the effect of therapeutic kinase inhibitors on cell survival. Mol Cancer Ther. 2005;4:257–70. - PubMed
-
- Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, Shaw YJ, Kulp SK, Chen CS. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–18. - PubMed
-
- Yacoub A, Park MA, Hanna D, Hong Y, Mitchell C, Pandya AP, Harada H, Powis G, Chen CS, Koumenis C, Grant S, Dent P. OSU-03012 promotes caspase-independent but PERK-, cathepsin B-, BID-and AIF-dependent killing of transformed cells. Mol Pharmacol. 2006;70:589–603. - PubMed
-
- Park MA, Yacoub A, Rahmani M, Zhang G, Hart L, Hagan MP, Calderwood SK, Sherman MY, Koumenis C, Spiegel S, Chen CS, Graf M, Curiel DT, et al. OSU-03012 stimulates PKR-like endoplasmic reticulum-dependent increases in 70-kDa heat shock protein expression, attenuating its lethal actions in transformed cells. Mol Pharmacol. 2008;73:1168–84. - PMC - PubMed
-
- Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee WC, Feng WC, Chen CS, Kuo ML, Cheng AL. OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res. 2008;68:9348–57. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

