Can roflumilast, a phosphodiesterase-4 inhibitor, improve clinical outcomes in patients with moderate-to-severe chronic obstructive pulmonary disease? A meta-analysis
- PMID: 26887407
- PMCID: PMC4756424
- DOI: 10.1186/s12931-016-0330-y
Can roflumilast, a phosphodiesterase-4 inhibitor, improve clinical outcomes in patients with moderate-to-severe chronic obstructive pulmonary disease? A meta-analysis
Abstract
Background: Effects of roflumilast on lung function, symptoms, acute exacerbation and adverse events in patients with chronic obstructive pulmonary disease (COPD) are controversial. We aimed to further clarify the efficacy and safety of roflumilast in treatment of moderate-to-severe COPD.
Methods: From 1946 to November 2015, we searched the Pubmed, Embase, Medline, Cochrane Central Register of Controlled Trials, ISI Web of Science and American College of Physician using "roflumilast" and "chronic obstructive pulmonary disease" or "COPD". Randomized controlled trials that reported forced expiratory volume in one second (FEV1), forced vital capacity (FVC), transition dyspnea index (TDI), St George's Respiratory Questionnaire (SGRQ), and incidence of COPD exacerbations and adverse events were eligible. We conducted the heterogeneities test and sensitivity analysis, and random-effects or fixed-effects model was applied to calculate risk ratio (RR) and mean difference (MD) for dichotomous and continuous data respectively. Cochrane systematic review software, Review Manager (RevMan), was used to test the hypothesis by Mann-Whitney U-test.
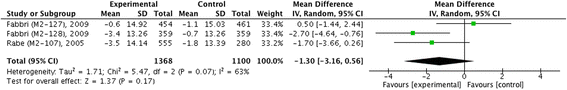
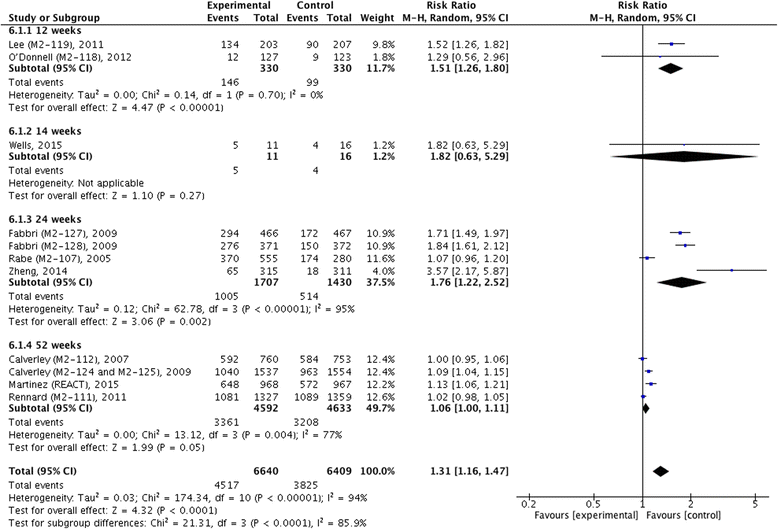
Results: Thirteen trials with a total of 14,563 patients were pooled in our final studies. Except for SGRQ (I (2) = 63 %, χ (2) = 1.71, P = 0.07) and adverse events (I (2) = 94 %, χ (2) = 0.03, P < 0.001), we did not find statistical heterogeneity in outcome measures. The pooled MD of pre- and post-bronchodilator FEV1 was 54.60 (95 % confidence interval (CI) 46.02 ~ 63.18) and 57.86 (95 % CI 49.80 ~ 65.91), and both showed significant improvement in patients with roflumilast (z = 12.47, P <0.001; z = 14.07, P < 0.001), so did in FVC (MD 90.37, 95 % CI 73.95 ~ 106.78, z = 10.79, P < 0.001). Significant alleviation of TDI (MD 0.30, 95 % CI 0.14 ~ 0.46, z = 3.67, P < 0.001) and decrease of acute exacerbation (RR 0.86, 95 % CI 0.81 ~ 0.91, z = 5.54, P < 0.001) were also identified in treatment of roflumilast, but without significant difference in SGRQ (MD -1.30, 95 % CI -3.16 ~ 0.56, z = 1.37, P = 0.17). Moreover, roflumilast significantly increased the incidence of adverse events compared with placebo (RR 1.31, 95 % CI 1.16 ~ 1.47, z = 4.32, P < 0.001).
Conclusions: Roflumilast can be considered as an alternative therapy in selective patients with moderate-to-severe COPD.
Figures









References
-
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (updated 2015). Global Initiative for Chronic Obstructive Lung Disease (GLOD). 2015. www.goldcopd.org. Accessed on February 18, 2015.
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

