The sexual identity of adult intestinal stem cells controls organ size and plasticity
- PMID: 26887495
- PMCID: PMC4800002
- DOI: 10.1038/nature16953
The sexual identity of adult intestinal stem cells controls organ size and plasticity
Abstract
Sex differences in physiology and disease susceptibility are commonly attributed to developmental and/or hormonal factors, but there is increasing realization that cell-intrinsic mechanisms play important and persistent roles. Here we use the Drosophila melanogaster intestine to investigate the nature and importance of cellular sex in an adult somatic organ in vivo. We find that the adult intestinal epithelium is a cellular mosaic of different sex differentiation pathways, and displays extensive sex differences in expression of genes with roles in growth and metabolism. Cell-specific reversals of the sexual identity of adult intestinal stem cells uncovers the key role this identity has in controlling organ size, reproductive plasticity and response to genetically induced tumours. Unlike previous examples of sexually dimorphic somatic stem cell activity, the sex differences in intestinal stem cell behaviour arise from intrinsic mechanisms that control cell cycle duration and involve a new doublesex- and fruitless-independent branch of the sex differentiation pathway downstream of transformer. Together, our findings indicate that the plasticity of an adult somatic organ is reversibly controlled by its sexual identity, imparted by a new mechanism that may be active in more tissues than previously recognized.
Figures


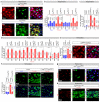
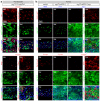
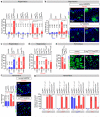
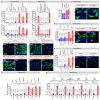


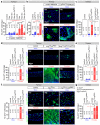
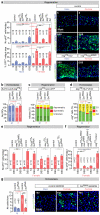
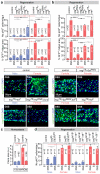
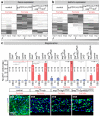
Comment in
-
Developmental biology: Females have a lot of guts.Nature. 2016 Feb 18;530(7590):289-90. doi: 10.1038/530289a. Nature. 2016. PMID: 26887491 No abstract available.
-
Sexual Dimorphism: How Female Cells Win the Race.Curr Biol. 2016 Mar 7;26(5):R212-5. doi: 10.1016/j.cub.2016.01.062. Curr Biol. 2016. PMID: 26954444
References
-
- Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987;50:739–747. - PubMed
ADDITIONAL REFERENCES
-
- Lu B, Ackerman L, Jan LY, Jan YN. Modes of protein movement that lead to the asymmetric localization of partner of Numb during Drosophila neuroblast division. Molecular cell. 1999;4:883–891. - PubMed
-
- Zielke N, et al. Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell reports. 2014;7:588–598. doi:10.1016/j.celrep.2014.03.020. - PubMed
-
- Ferveur JF, Stortkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. - PubMed
-
- Lee G, Hall JC, Park JH. Doublesex gene expression in the central nervous system of Drosophila melanogaster. Journal of neurogenetics. 2002;16:229–248. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

