Path to Collagenolysis: COLLAGEN V TRIPLE-HELIX MODEL BOUND PRODUCTIVELY AND IN ENCOUNTERS BY MATRIX METALLOPROTEINASE-12
- PMID: 26887942
- PMCID: PMC4824997
- DOI: 10.1074/jbc.M115.703124
Path to Collagenolysis: COLLAGEN V TRIPLE-HELIX MODEL BOUND PRODUCTIVELY AND IN ENCOUNTERS BY MATRIX METALLOPROTEINASE-12
Abstract
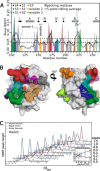
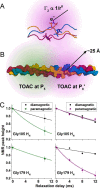
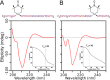
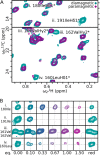
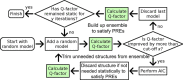
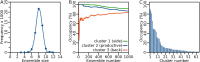
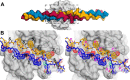
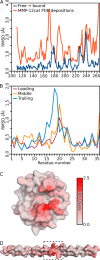
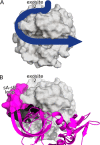
Collagenolysis is essential in extracellular matrix homeostasis, but its structural basis has long been shrouded in mystery. We have developed a novel docking strategy guided by paramagnetic NMR that positions a triple-helical collagen V mimic (synthesized with nitroxide spin labels) in the active site of the catalytic domain of matrix metalloproteinase-12 (MMP-12 or macrophage metalloelastase) primed for catalysis. The collagenolytically productive complex forms by utilizing seven distinct subsites that traverse the entire length of the active site. These subsites bury ∼1,080 Å(2)of surface area, over half of which is contributed by the trailing strand of the synthetic collagen V mimic, which also appears to ligate the catalytic zinc through the glycine carbonyl oxygen of its scissile G∼VV triplet. Notably, the middle strand also occupies the full length of the active site where it contributes extensive interfacial contacts with five subsites. This work identifies, for the first time, the productive and specific interactions of a collagen triple helix with an MMP catalytic site. The results uniquely demonstrate that the active site of the MMPs is wide enough to accommodate two strands from collagen triple helices. Paramagnetic relaxation enhancements also reveal an extensive array of encounter complexes that form over a large part of the catalytic domain. These transient complexes could possibly facilitate the formation of collagenolytically active complexes via directional Brownian tumbling.
Keywords: collagen; docking; encounter complex; matrix metalloproteinase (MMP); nuclear magnetic resonance (NMR); paramagnetic relaxation enhancement (PRE); protein-protein interaction.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Fields G. B. (1991) A model for interstitial collagen catabolism by mammalian collagenases. J. Theor. Biol. 153, 585–602 - PubMed
-
- Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., and Friedl P. (2007) Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9, 893–904 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

