Evaluation of an antenatal acupuncture intervention as an adjunct therapy for antenatal depression (AcuAnteDep): study protocol for a pragmatic randomised controlled trial
- PMID: 26887958
- PMCID: PMC4758005
- DOI: 10.1186/s13063-016-1204-9
Evaluation of an antenatal acupuncture intervention as an adjunct therapy for antenatal depression (AcuAnteDep): study protocol for a pragmatic randomised controlled trial
Abstract
Background: Depressed pregnant women face difficulty navigating a course between the potentially serious consequences of leaving depression untreated and significant limitations associated with conventional therapies, such as foetal toxicity and teratogenicity. Preliminary evidence is suggestive that acupuncture may provide a safe and effective alternative treatment option for antenatal depression; however, additional research is required. The purpose of this study is to further investigate this treatment possibility, with an additional examination of a potential biomechanistic acupuncture effect.
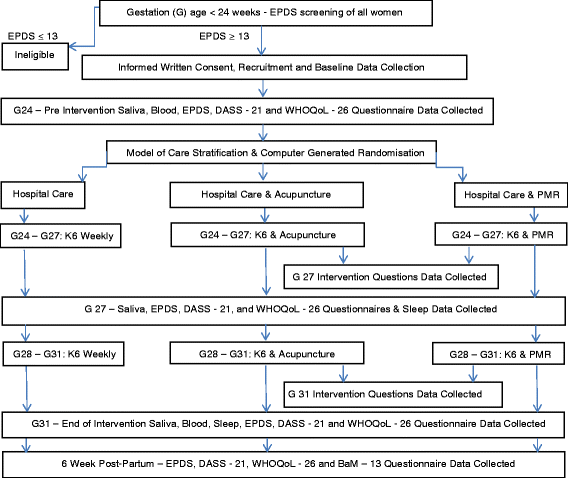
Methods/design: In this pragmatic randomised controlled trial, we will compare individually tailored, flexible antenatal depression-oriented acupuncture with equivalent attention progressive muscle relaxation and routine antenatal depression hospital care. Eligible women at 24 weeks of gestation with Edinburgh Postnatal Depression Scale scores of 13 or more will be recruited from 2 antenatal clinics in South Western Sydney, Australia. The recruitment goal of 96 is powered to demonstrate a significant difference in Edinburgh Postnatal Depression Scale score severity between acupuncture and usual care, with intervention groups receiving weekly 1-h treatments for 8 weeks from 24 to 31 weeks of gestation. Mental health and quality-of-life assessments will occur at study commencement, intervention weeks 4 and 8 and 6 weeks post-natally via the collection of completed Edinburgh Postnatal Depression Scale scores, Depression, Stress and Anxiety Scale scores and World Health Organisation Quality of Life Scale scores. Adjustment to mothering will also be evaluated at 6 weeks post-natally using the Being a Mother Scale. A putative biomechanistic effect of acupuncture on the oxytocinergic system will additionally be examined by comparing baseline salivary hormone levels with those measured at intervention weeks 4 and 8, as well as leucocyte oxytocin receptor expression at baseline and intervention week 8.
Discussion: Ethical approval was received in February 2015, and recruitment is underway and expected to be completed in July 2016.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12615000250538, Registered on 19 March 2015.
Figures
Similar articles
-
Investigation of Hypothalamic Pituitary Adrenal Axis and Oxytocinergic System Changes in a Pragmatic Randomized Controlled Feasibility Trial of Acupuncture for Antenatal Depression.J Integr Complement Med. 2024 Feb;30(2):173-184. doi: 10.1089/jicm.2023.0012. Epub 2023 Aug 11. J Integr Complement Med. 2024. PMID: 37566543 Clinical Trial.
-
Depression: an exploratory parallel-group randomised controlled trial of Antenatal guided self help for WomeN (DAWN): study protocol for a randomised controlled trial.Trials. 2016 Oct 18;17(1):503. doi: 10.1186/s13063-016-1632-6. Trials. 2016. PMID: 27756349 Free PMC article. Clinical Trial.
-
The feasibility of acupuncture as an adjunct intervention for antenatal depression: a pragmatic randomised controlled trial.J Affect Disord. 2020 Oct 1;275:82-93. doi: 10.1016/j.jad.2020.05.089. Epub 2020 May 24. J Affect Disord. 2020. PMID: 32658830 Clinical Trial.
-
The effect of complementary medicines and therapies on maternal anxiety and depression in pregnancy: A systematic review and meta-analysis.J Affect Disord. 2019 Feb 15;245:428-439. doi: 10.1016/j.jad.2018.11.054. Epub 2018 Nov 6. J Affect Disord. 2019. PMID: 30423471
-
Treatment of Depression with Acupuncture Based on Pathophysiological Mechanism.Int J Gen Med. 2024 Jan 31;17:347-357. doi: 10.2147/IJGM.S448031. eCollection 2024. Int J Gen Med. 2024. PMID: 38314195 Free PMC article. Review.
Cited by
-
Effectiveness of Acupuncture Used for the Management of Postpartum Depression: A Systematic Review and Meta-Analysis.Biomed Res Int. 2019 Mar 20;2019:6597503. doi: 10.1155/2019/6597503. eCollection 2019. Biomed Res Int. 2019. PMID: 31016194 Free PMC article.
-
Fecal microbiota as a predictor of acupuncture responses in patients with postpartum depressive disorder.Front Cell Infect Microbiol. 2023 Nov 20;13:1228940. doi: 10.3389/fcimb.2023.1228940. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38053532 Free PMC article.
References
-
- Choate LH, Gintner GG. Prenatal depression: best practice guidelines for diagnosis and treatment. J Couns Dev. 2011;89(3):373–81. doi: 10.1002/j.1556-6678.2011.tb00102.x. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

