Testing the hypothesis of neurodegeneracy in respiratory network function with a priori transected arterially perfused brain stem preparation of rat
- PMID: 26888109
- PMCID: PMC4922475
- DOI: 10.1152/jn.01073.2015
Testing the hypothesis of neurodegeneracy in respiratory network function with a priori transected arterially perfused brain stem preparation of rat
Abstract
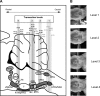
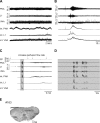
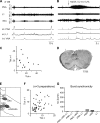
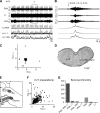
Degeneracy of respiratory network function would imply that anatomically discrete aspects of the brain stem are capable of producing respiratory rhythm. To test this theory we a priori transected brain stem preparations before reperfusion and reoxygenation at 4 rostrocaudal levels: 1.5 mm caudal to obex (n = 5), at obex (n = 5), and 1.5 (n = 7) and 3 mm (n = 6) rostral to obex. The respiratory activity of these preparations was assessed via recordings of phrenic and vagal nerves and lumbar spinal expiratory motor output. Preparations with a priori transection at level of the caudal brain stem did not produce stable rhythmic respiratory bursting, even when the arterial chemoreceptors were stimulated with sodium cyanide (NaCN). Reperfusion of brain stems that preserved the pre-Bötzinger complex (pre-BötC) showed spontaneous and sustained rhythmic respiratory bursting at low phrenic nerve activity (PNA) amplitude that occurred simultaneously in all respiratory motor outputs. We refer to this rhythm as the pre-BötC burstlet-type rhythm. Conserving circuitry up to the pontomedullary junction consistently produced robust high-amplitude PNA at lower burst rates, whereas sequential motor patterning across the respiratory motor outputs remained absent. Some of the rostrally transected preparations expressed both burstlet-type and regular PNA amplitude rhythms. Further analysis showed that the burstlet-type rhythm and high-amplitude PNA had 1:2 quantal relation, with burstlets appearing to trigger high-amplitude bursts. We conclude that no degenerate rhythmogenic circuits are located in the caudal medulla oblongata and confirm the pre-BötC as the primary rhythmogenic kernel. The absence of sequential motor patterning in a priori transected preparations suggests that pontine circuits govern respiratory pattern formation.
Keywords: breathing; medulla oblongata; neural circuits; respiratory pattern; respiratory rhythm generation.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol 143: 105–114, 2004. - PubMed
-
- Aoki M, Mori S, Kawahara K, Watanabe H, Ebata N. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res 202: 51–63, 1980. - PubMed
-
- Baghdadwala MI, Duchcherer M, Trask WM, Gray PA, Wilson RJ. Diving into the mammalian swamp of respiratory rhythm generation with the bullfrog. Respir Physiol Neurobiol 14: 37–51, 2016. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

