Structure and functions of angiotensinogen
- PMID: 26888118
- PMCID: PMC4935807
- DOI: 10.1038/hr.2016.17
Structure and functions of angiotensinogen
Erratum in
-
Corrigendum: Structure and functions of angiotensinogen.Hypertens Res. 2016 Nov;39(11):827. doi: 10.1038/hr.2016.106. Hypertens Res. 2016. PMID: 27818490 Free PMC article. No abstract available.
Abstract
Angiotensinogen (AGT) is the sole precursor of all angiotensin peptides. Although AGT is generally considered as a passive substrate of the renin-angiotensin system, there is accumulating evidence that the regulation and functions of AGT are intricate. Understanding the diversity of AGT properties has been enhanced by protein structural analysis and animal studies. In addition to whole-body genetic deletion, AGT can be regulated in vivo by cell-specific procedures, adeno-associated viral approaches and antisense oligonucleotides. Indeed, the availability of these multiple manipulations of AGT in vivo has provided new insights into the multifaceted roles of AGT. In this review, the combination of structural and functional studies is highlighted to focus on the increasing recognition that AGT exerts effects beyond being a sole provider of angiotensin peptides.
Figures

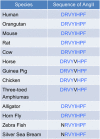

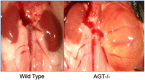
References
-
- Streatfeild-James RM, Williamson D, Pike RN, Tewksbury D, Carrell RW, Coughlin PB. Angiotensinogen cleavage by renin: importance of a structurally constrained N-terminus. FEBS Lett 1998; 436: 267–270. - PubMed
-
- Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo MS, Takahashi S, Hatae T, Kajiwara N, Yagami K, Murakami K. Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem 1993; 268: 11617–11621. - PubMed
-
- Yang G, Merrill DC, Thompson MW, Robillard JE, Sigmund CD. Functional expression of the human angiotensinogen gene in transgenic mice. J Biol Chem 1994; 269: 32497–32502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

