Galectin-1 drives lymphoma CD20 immunotherapy resistance: validation of a preclinical system to identify resistance mechanisms
- PMID: 26888257
- PMCID: PMC4832507
- DOI: 10.1182/blood-2015-11-681130
Galectin-1 drives lymphoma CD20 immunotherapy resistance: validation of a preclinical system to identify resistance mechanisms
Abstract
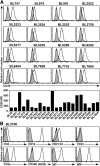
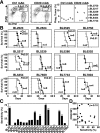
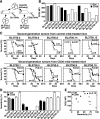
Non-Hodgkin lymphoma (NHL) is the most commonly diagnosed hematologic cancer of adults in the United States, with the vast majority of NHLs deriving from malignant B lymphocytes that express cell surface CD20. CD20 immunotherapy (rituximab) is widely used to treat NHL, even though the initial effectiveness of rituximab varies widely among patients and typically wanes over time. The mechanisms through which lymphomas initially resist or gain resistance to immunotherapy are not well established. To address this, a preclinical mouse model system was developed to comprehensively identify lymphoma transcriptomic changes that confer resistance to CD20 immunotherapy. The generation of spontaneous primary and familial lymphomas revealed that sensitivity to CD20 immunotherapy was not regulated by differences in CD20 expression, prior exposure to CD20 immunotherapy, or serial in vivo passage. An unbiased forward exome screen of these primary lymphomas was used to validate the utility of this expansive lymphoma cohort, which revealed that increased lymphoma galectin-1 (Gal-1) expression strongly correlated with resistance to immunotherapy. Genetically induced lymphoma Gal-1 expression ablated antibody-dependent lymphoma phagocytosis in vitro and lymphoma sensitivity to CD20 immunotherapy in vivo. Human NHLs also express elevated Gal-1 compared with nonmalignant lymphocytes, demonstrating the ability of this preclinical model system to identify molecular targets that could be relevant to human therapy. This study therefore established a powerful preclinical model system that permits the comprehensive identification of the dynamic lymphoma molecular network that drives resistance to immunotherapy.
© 2016 by The American Society of Hematology.
Figures




References
-
- American Cancer Society. Atlanta, GA: American Cancer Society; 2014. Cancer Facts & Figures 2014.
-
- Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63(6):1424–1433. - PubMed
-
- Maloney DG, Grillo-López AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15(10):3266–3274. - PubMed
-
- Grillo-López AJ. Rituximab (Rituxan/MabThera): the first decade (1993-2003). Expert Rev Anticancer Ther. 2003;3(6):767–779. - PubMed
-
- Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18(17):3135–3143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

