Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study
- PMID: 26888382
- PMCID: PMC4772785
- DOI: 10.1136/bmj.i581
Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study
Abstract
Objective: To determine whether the use of incretin based drugs compared with sulfonylureas is associated with an increased risk of incident pancreatic cancer in people with type 2 diabetes.
Design: Population based cohort.
Setting: Large, international, multicentre study combining the health records from six participating sites in Canada, the United States, and the United Kingdom.
Participants: A cohort of 972,384 patients initiating antidiabetic drugs between 1 January 2007 and 30 June 2013, with follow-up until 30 June 2014.
Main outcome measures: Within each cohort we conducted nested case-control analyses, where incident cases of pancreatic cancer were matched with up to 20 controls on sex, age, cohort entry date, duration of treated diabetes, and duration of follow-up. Hazard ratios and 95% confidence intervals for incident pancreatic cancer were estimated, comparing use of incretin based drugs with use of sulfonylureas, with drug use lagged by one year for latency purposes. Secondary analyses assessed whether the risk varied by class (dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists) or by duration of use (cumulative duration of use and time since treatment initiation). Site specific hazard ratios were pooled using random effects models.
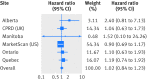
Results: During 2,024,441 person years of follow-up (median follow-up ranging from 1.3 to 2.8 years; maximum 8 years), 1221 patients were newly diagnosed as having pancreatic cancer (incidence rate 0.60 per 1000 person years). Compared with sulfonylureas, incretin based drugs were not associated with an increased risk of pancreatic cancer (pooled adjusted hazard ratio 1.02, 95% confidence interval 0.84 to 1.23). Similarly, the risk did not vary by class and evidence of a duration-response relation was lacking.
Conclusions: In this large, population based study the use of incretin based drugs was not associated with an increased risk of pancreatic cancer compared with sulfonylureas. Although this potential adverse drug reaction will need to be monitored long term owing to the latency of the cancer, these findings provide some reassurance on the safety of incretin based drugs.
Trial registration: ClinicalTrials.gov NCT02475499.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


Comment in
-
The safety of incretin based drug treatments for type 2 diabetes.BMJ. 2016 Feb 17;352:i801. doi: 10.1136/bmj.i801. BMJ. 2016. PMID: 26888024 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
