Abnormal tau induces cognitive impairment through two different mechanisms: synaptic dysfunction and neuronal loss
- PMID: 26888634
- PMCID: PMC4757872
- DOI: 10.1038/srep20833
Abnormal tau induces cognitive impairment through two different mechanisms: synaptic dysfunction and neuronal loss
Abstract
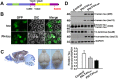
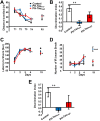
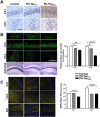
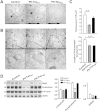
The hyperphosphorylated microtubule-associated protein tau is present in several neurodegenerative diseases, although the causal relationship remains elusive. Few mouse models used to study Alzheimer-like dementia target tau phosphorylation. We created an inducible pseudophosphorylated tau (Pathological Human Tau, PH-Tau) mouse model to study the effect of conformationally modified tau in vivo. Leaky expression resulted in two levels of PH-Tau: low basal level and higher upon induction (4% and 14% of the endogenous tau, respectively). Unexpectedly, low PH-Tau resulted in significant cognitive deficits, decrease in the number of synapses (seen by EM in the CA1 region), reduction of synaptic proteins, and localization to the nucleus. Induction of PH-Tau triggered neuronal death (60% in CA3), astrocytosis, and loss of the processes in CA1. These findings suggest, that phosphorylated tau is sufficient to induce neurodegeneration and that two different mechanisms can induce cognitive impairment depending on the levels of PH-Tau expression.
Figures


References
-
- Lee V. M., Goedert M. & Trojanowski J. Q. Neurodegenerative tauopathies. Annu Rev Neurosci 24, 1121–1159 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

