Dopaminergic Contributions to Vocal Learning
- PMID: 26888928
- PMCID: PMC4756153
- DOI: 10.1523/JNEUROSCI.3883-15.2016
Dopaminergic Contributions to Vocal Learning
Abstract
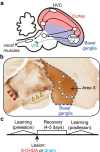
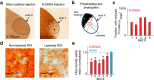
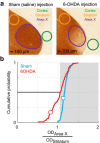
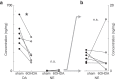
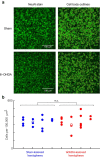
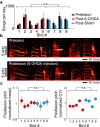
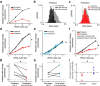
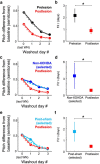
Although the brain relies on auditory information to calibrate vocal behavior, the neural substrates of vocal learning remain unclear. Here we demonstrate that lesions of the dopaminergic inputs to a basal ganglia nucleus in a songbird species (Bengalese finches, Lonchura striata var. domestica) greatly reduced the magnitude of vocal learning driven by disruptive auditory feedback in a negative reinforcement task. These lesions produced no measureable effects on the quality of vocal performance or the amount of song produced. Our results suggest that dopaminergic inputs to the basal ganglia selectively mediate reinforcement-driven vocal plasticity. In contrast, dopaminergic lesions produced no measurable effects on the birds' ability to restore song acoustics to baseline following the cessation of reinforcement training, suggesting that different forms of vocal plasticity may use different neural mechanisms.
Significance statement: During skill learning, the brain relies on sensory feedback to improve motor performance. However, the neural basis of sensorimotor learning is poorly understood. Here, we investigate the role of the neurotransmitter dopamine in regulating vocal learning in the Bengalese finch, a songbird with an extremely precise singing behavior that can nevertheless be reshaped dramatically by auditory feedback. Our findings show that reduction of dopamine inputs to a region of the songbird basal ganglia greatly impairs vocal learning but has no detectable effect on vocal performance. These results suggest a specific role for dopamine in regulating vocal plasticity.
Keywords: Bengalese finch; basal ganglia; dopamine; negative reinforcement; songbird; vocal learning.
Copyright © 2016 the authors 0270-6474/16/362176-14$15.00/0.
Figures
Similar articles
-
Dopamine Depletion Affects Vocal Acoustics and Disrupts Sensorimotor Adaptation in Songbirds.eNeuro. 2019 Jun 12;6(3):ENEURO.0190-19.2019. doi: 10.1523/ENEURO.0190-19.2019. Print 2019 May/Jun. eNeuro. 2019. PMID: 31126913 Free PMC article.
-
Shared mechanisms of auditory and non-auditory vocal learning in the songbird brain.Elife. 2022 Sep 15;11:e75691. doi: 10.7554/eLife.75691. Elife. 2022. PMID: 36107757 Free PMC article.
-
Mechanisms and time course of vocal learning and consolidation in the adult songbird.J Neurophysiol. 2011 Oct;106(4):1806-21. doi: 10.1152/jn.00311.2011. Epub 2011 Jul 6. J Neurophysiol. 2011. PMID: 21734110 Free PMC article.
-
Variability in action: Contributions of a songbird cortical-basal ganglia circuit to vocal motor learning and control.Neuroscience. 2015 Jun 18;296:39-47. doi: 10.1016/j.neuroscience.2014.10.010. Epub 2014 Oct 18. Neuroscience. 2015. PMID: 25445191 Review.
-
Two neural streams, one voice: pathways for theme and variation in the songbird brain.Neuroscience. 2014 Sep 26;277:806-17. doi: 10.1016/j.neuroscience.2014.07.061. Epub 2014 Aug 8. Neuroscience. 2014. PMID: 25106128 Review.
Cited by
-
Actor-critic reinforcement learning in the songbird.Curr Opin Neurobiol. 2020 Dec;65:1-9. doi: 10.1016/j.conb.2020.08.005. Epub 2020 Sep 6. Curr Opin Neurobiol. 2020. PMID: 32898752 Free PMC article. Review.
-
What Is the Role of Thalamostriatal Circuits in Learning Vocal Sequences?Front Neural Circuits. 2021 Sep 22;15:724858. doi: 10.3389/fncir.2021.724858. eCollection 2021. Front Neural Circuits. 2021. PMID: 34630047 Free PMC article.
-
A common neural circuit mechanism for internally guided and externally reinforced forms of motor learning.Nat Neurosci. 2018 Apr;21(4):589-597. doi: 10.1038/s41593-018-0092-6. Epub 2018 Feb 26. Nat Neurosci. 2018. PMID: 29483664 Free PMC article.
-
Unilateral vocal nerve resection alters neurogenesis in the avian song system in a region-specific manner.PLoS One. 2021 Aug 31;16(8):e0256709. doi: 10.1371/journal.pone.0256709. eCollection 2021. PLoS One. 2021. PMID: 34464400 Free PMC article.
-
Rules and mechanisms for efficient two-stage learning in neural circuits.Elife. 2017 Apr 4;6:e20944. doi: 10.7554/eLife.20944. Elife. 2017. PMID: 28374674 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
