Distinct β Band Oscillatory Networks Subserving Motor and Cognitive Control during Gait Adaptation
- PMID: 26888931
- PMCID: PMC6602036
- DOI: 10.1523/JNEUROSCI.3543-15.2016
Distinct β Band Oscillatory Networks Subserving Motor and Cognitive Control during Gait Adaptation
Abstract
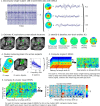
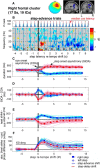
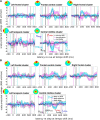
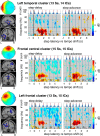
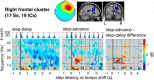
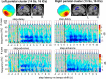
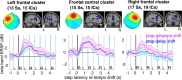
Everyday locomotion and obstacle avoidance requires effective gait adaptation in response to sensory cues. Many studies have shown that efficient motor actions are associated with μ rhythm (8-13 Hz) and β band (13-35 Hz) local field desynchronizations in sensorimotor and parietal cortex, whereas a number of cognitive task studies have reported higher behavioral accuracy to be associated with increases in β band power in prefrontal and sensory cortex. How these two distinct patterns of β band oscillations interplay during gait adaptation, however, has not been established. Here we recorded 108 channel EEG activity from 18 participants (10 males, 22-35 years old) attempting to walk on a treadmill in synchrony with a series of pacing cue tones, and quickly adapting their step rate and length to sudden shifts in pacing cue tempo. Independent component analysis parsed each participant's EEG data into maximally independent component (IC) source processes, which were then grouped across participants into distinct spatial/spectral clusters. Following cue tempo shifts, mean β band power was suppressed for IC sources in central midline and parietal regions, whereas mean β band power increased in IC sources in or near medial prefrontal and dorsolateral prefrontal cortex. In the right dorsolateral prefrontal cortex IC cluster, the β band power increase was stronger during (more effortful) step shortening than during step lengthening. These results thus show that two distinct patterns of β band activity modulation accompany gait adaptations: one likely serving movement initiation and execution; and the other, motor control and inhibition.
Significance statement: Understanding brain dynamics supporting gait adaptation is crucial for understanding motor deficits in walking, such as those associated with aging, stroke, and Parkinson's. Only a few electromagnetic brain imaging studies have examined neural correlates of human upright walking. Here, application of independent component analysis to EEG data recorded during treadmill walking allowed us to uncover two distinct β band oscillatory cortical networks that are active during gait adaptation to shifts in the tempo of an auditory pacing cue: (8-13 Hz) μ rhythm and (13-35 Hz) β band power decreases in central and parietal cortex and (14-20 Hz) β band power increases in frontal brain areas. These results provide a fuller framework for electrophysiological studies of cortical gait control and its disorders.
Keywords: EEG; gait adaptation; independent component analysis; motor inhibition; rhythmic auditory cueing; β band oscillations.
Copyright © 2016 the authors 0270-6474/16/362212-15$15.00/0.
Figures



Similar articles
-
Electrocortical activity correlated with locomotor adaptation during split-belt treadmill walking.J Physiol. 2023 Sep;601(17):3921-3944. doi: 10.1113/JP284505. Epub 2023 Jul 31. J Physiol. 2023. PMID: 37522890 Free PMC article.
-
Trial-by-trial source-resolved EEG responses to gait task challenges predict subsequent step adaptation.Neuroimage. 2019 Oct 1;199:691-703. doi: 10.1016/j.neuroimage.2019.06.018. Epub 2019 Jun 7. Neuroimage. 2019. PMID: 31181332 Free PMC article.
-
Spatially Distinct Beta-Band Activities Reflect Implicit Sensorimotor Adaptation and Explicit Re-aiming Strategy.J Neurosci. 2020 Mar 18;40(12):2498-2509. doi: 10.1523/JNEUROSCI.1862-19.2020. Epub 2020 Feb 7. J Neurosci. 2020. PMID: 32034068 Free PMC article.
-
Sensorimotor and cognitive involvement of the beta-gamma oscillation in the frontal N30 component of somatosensory evoked potentials.Neuropsychologia. 2015 Dec;79(Pt B):215-22. doi: 10.1016/j.neuropsychologia.2015.04.033. Epub 2015 May 19. Neuropsychologia. 2015. PMID: 26002756 Review.
-
Spontaneous Beta Band Rhythms in the Predictive Coding of Natural Stimuli.Neuroscientist. 2021 Apr;27(2):184-201. doi: 10.1177/1073858420928988. Epub 2020 Jun 15. Neuroscientist. 2021. PMID: 32538310 Free PMC article. Review.
Cited by
-
Brain-Machine Neurofeedback: Robotics or Electrical Stimulation?Front Bioeng Biotechnol. 2020 Jul 7;8:639. doi: 10.3389/fbioe.2020.00639. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32733860 Free PMC article.
-
Weakened Effective Connectivity Related to Electroacupuncture in Stroke Patients with Prolonged Flaccid Paralysis: An EEG Pilot Study.Neural Plast. 2021 Mar 9;2021:6641506. doi: 10.1155/2021/6641506. eCollection 2021. Neural Plast. 2021. PMID: 33777135 Free PMC article. Clinical Trial.
-
Challenge for Affective Brain-Computer Interfaces: Non-stationary Spatio-spectral EEG Oscillations of Emotional Responses.Front Hum Neurosci. 2019 Oct 30;13:366. doi: 10.3389/fnhum.2019.00366. eCollection 2019. Front Hum Neurosci. 2019. PMID: 31736727 Free PMC article.
-
Electrocortical activity correlated with locomotor adaptation during split-belt treadmill walking.J Physiol. 2023 Sep;601(17):3921-3944. doi: 10.1113/JP284505. Epub 2023 Jul 31. J Physiol. 2023. PMID: 37522890 Free PMC article.
-
Balance task difficulty affects postural sway and cortical activity in healthy adolescents.Exp Brain Res. 2020 May;238(5):1323-1333. doi: 10.1007/s00221-020-05810-1. Epub 2020 Apr 23. Exp Brain Res. 2020. PMID: 32328673 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
