Guidance of Axons by Local Coupling of Retrograde Flow to Point Contact Adhesions
- PMID: 26888936
- PMCID: PMC4756158
- DOI: 10.1523/JNEUROSCI.2645-15.2016
Guidance of Axons by Local Coupling of Retrograde Flow to Point Contact Adhesions
Abstract
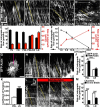
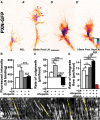
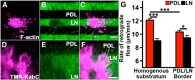
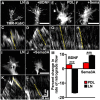
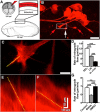
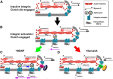
Growth cones interact with the extracellular matrix (ECM) through integrin receptors at adhesion sites termed point contacts. Point contact adhesions link ECM proteins to the actin cytoskeleton through numerous adaptor and signaling proteins. One presumed function of growth cone point contacts is to restrain or "clutch" myosin-II-based filamentous actin (F-actin) retrograde flow (RF) to promote leading edge membrane protrusion. In motile non-neuronal cells, myosin-II binds and exerts force upon actin filaments at the leading edge, where clutching forces occur. However, in growth cones, it is unclear whether similar F-actin-clutching forces affect axon outgrowth and guidance. Here, we show in Xenopus spinal neurons that RF is reduced in rapidly migrating growth cones on laminin (LN) compared with non-integrin-binding poly-d-lysine (PDL). Moreover, acute stimulation with LN accelerates axon outgrowth over a time course that correlates with point contact formation and reduced RF. These results suggest that RF is restricted by the assembly of point contacts, which we show occurs locally by two-channel imaging of RF and paxillin. Further, using micropatterns of PDL and LN, we demonstrate that individual growth cones have differential RF rates while interacting with two distinct substrata. Opposing effects on RF rates were also observed in growth cones treated with chemoattractive and chemorepulsive axon guidance cues that influence point contact adhesions. Finally, we show that RF is significantly attenuated in vivo, suggesting that it is restrained by molecular clutching forces within the spinal cord. Together, our results suggest that local clutching of RF can control axon guidance on ECM proteins downstream of axon guidance cues.
Significance statement: Here, we correlate point contact adhesions directly with clutching of filamentous actin retrograde flow (RF), which our findings strongly suggest guides developing axons. Acute assembly of new point contact adhesions is temporally and spatially linked to attenuation of RF at sites of forward membrane protrusion. Importantly, clutching of RF is modulated by extracellular matrix (ECM) proteins and soluble axon guidance cues, suggesting that it may regulate axon guidance in vivo. Consistent with this notion, we found that RF rates of spinal neuron growth cones were slower in vivo than what was observed in vitro. Together, our study provides the best evidence that growth cone-ECM adhesions clutch RF locally to guide axons in vivo.
Keywords: actin retrograde flow; axon guidance; growth cone; kabiramide C; molecular clutch; point contact adhesion.
Copyright © 2016 the authors 0270-6474/16/362267-16$15.00/0.
Figures


Similar articles
-
Calpain-Mediated Proteolysis of Talin and FAK Regulates Adhesion Dynamics Necessary for Axon Guidance.J Neurosci. 2017 Feb 8;37(6):1568-1580. doi: 10.1523/JNEUROSCI.2769-16.2016. Epub 2017 Jan 9. J Neurosci. 2017. PMID: 28069919 Free PMC article.
-
Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding.Nat Neurosci. 2006 Oct;9(10):1274-83. doi: 10.1038/nn1762. Epub 2006 Sep 3. Nat Neurosci. 2006. PMID: 16964253
-
Regulation of ECM degradation and axon guidance by growth cone invadosomes.Development. 2015 Feb 1;142(3):486-96. doi: 10.1242/dev.108266. Epub 2015 Jan 6. Development. 2015. PMID: 25564649 Free PMC article.
-
The clutch hypothesis revisited: ascribing the roles of actin-associated proteins in filopodial protrusion in the nerve growth cone.J Neurobiol. 2000 Aug;44(2):114-25. doi: 10.1002/1097-4695(200008)44:2<114::aid-neu3>3.0.co;2-8. J Neurobiol. 2000. PMID: 10934316 Review.
-
Cell adhesion and invasion mechanisms that guide developing axons.Curr Opin Neurobiol. 2016 Aug;39:77-85. doi: 10.1016/j.conb.2016.04.012. Epub 2016 Apr 29. Curr Opin Neurobiol. 2016. PMID: 27135389 Free PMC article. Review.
Cited by
-
Environmental Elasticity Regulates Cell-type Specific RHOA Signaling and Neuritogenesis of Human Neurons.Stem Cell Reports. 2019 Dec 10;13(6):1006-1021. doi: 10.1016/j.stemcr.2019.10.008. Epub 2019 Nov 7. Stem Cell Reports. 2019. PMID: 31708476 Free PMC article.
-
Combinatorial nanodot stripe assay to systematically study cell haptotaxis.Microsyst Nanoeng. 2020 Dec 14;6:114. doi: 10.1038/s41378-020-00223-0. eCollection 2020. Microsyst Nanoeng. 2020. PMID: 33365138 Free PMC article.
-
Axon growth regulation by a bistable molecular switch.Proc Biol Sci. 2018 Apr 25;285(1877):20172618. doi: 10.1098/rspb.2017.2618. Proc Biol Sci. 2018. PMID: 29669897 Free PMC article.
-
An Integrated Cytoskeletal Model of Neurite Outgrowth.Front Cell Neurosci. 2018 Nov 26;12:447. doi: 10.3389/fncel.2018.00447. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30534055 Free PMC article. Review.
-
The Emerging Role of Mechanics in Synapse Formation and Plasticity.Front Cell Neurosci. 2018 Dec 6;12:483. doi: 10.3389/fncel.2018.00483. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30574071 Free PMC article. Review.
References
-
- Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
