Rate of initial highly active anti-retroviral therapy regimen change and its predictors among adult HIV patients at University of Gondar Referral Hospital, Northwest Ethiopia: a retrospective follow up study
- PMID: 26889204
- PMCID: PMC4756418
- DOI: 10.1186/s12981-016-0095-x
Rate of initial highly active anti-retroviral therapy regimen change and its predictors among adult HIV patients at University of Gondar Referral Hospital, Northwest Ethiopia: a retrospective follow up study
Abstract
Background: Regimen change is a major challenge for the sustainability of human immunodeficiency virus (HIV) treatment program. In a resource limited setting where treatment options are limited, designing strategies to increase the durability of original regimen are essential. However, information's on rate of initial regimen change and its predictors is scarce in Ethiopia. Therefore, the purpose of this study was to assess the rate of initial highly active anti retroviral therapy (HAART) regimen change and its predictors among adult HIV patients at the University of Gondar Referral Hospital, Northwest Ethiopia.
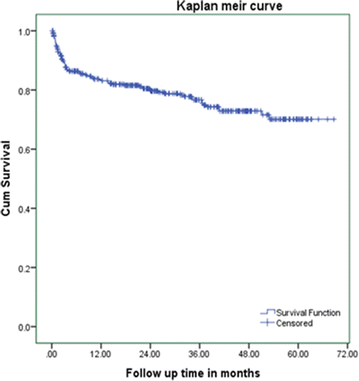
Methods: An institutional based retrospective follow up study was conducted among 410 adult HIV patients started HAART from January 2010 to December 2014. Simple random sampling technique was used to select patient records using computer generated random number. Data were collected from patient chart using data extraction tool. The Kaplan-Meier curve was used to estimate the median duration of regimen change. Life table was used to estimate the cumulative survival for initial regimen change and log rank test to compare regimen change survival curves between the different categories of explanatory variables. Bivariate and multivariate Cox proportional hazard model were used to identify predictors of initial regimen change.
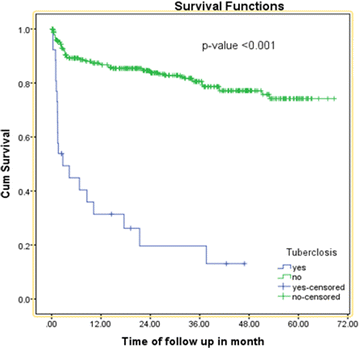
Results: The overall incidence rate of initial regimen change was 10.11 (95 % CI 8.29, 12.6) per 100 person years (PY). Baseline WHO clinical stage III (AHR = 1.92, 95 % CI 1.12-3.35), occurrence of tuberculosis (TB) on the initial regimen (AHR = 8.33, 95 % CI 4.47-15.53), side effect on the initial regimen (AHR = 25.27, 95 % CI 15.12-42.00) and co-medication with ART (AHR = 2.5, 95 % CI 1.46-4.34) were significant predictors of initial regimen change.
Conclusions: The rate of initial HAART regimen change was found to be high. Having WHO clinical stage III, co-medication with ART, occurrence of tuberculosis and side effect on initial regimen were independent predictors of regimen change. Hence, close follow-up and screening of patient for side effect and tuberculosis is important.
Keywords: Gondar; HIV infection; Rate; Regimen change; Survival analysis.
Figures
Similar articles
-
Time to initial highly active antiretroviral therapy discontinuation and its predictors among HIV patients in Felege Hiwot comprehensive specialized hospital: a retrospective cohort study.AIDS Res Ther. 2021 Dec 4;18(1):93. doi: 10.1186/s12981-021-00418-z. AIDS Res Ther. 2021. PMID: 34863200 Free PMC article.
-
The effect of incident tuberculosis on immunological response of HIV patients on highly active anti-retroviral therapy at the university of Gondar hospital, northwest Ethiopia: a retrospective follow-up study.BMC Infect Dis. 2014 Aug 27;14:468. doi: 10.1186/1471-2334-14-468. BMC Infect Dis. 2014. PMID: 25164855 Free PMC article.
-
Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. A retrospective follow-up study.PLoS One. 2018 May 22;13(5):e0197145. doi: 10.1371/journal.pone.0197145. eCollection 2018. PLoS One. 2018. PMID: 29787596 Free PMC article.
-
Magnitude and causes of first-line antiretroviral therapy regimen changes among HIV patients in Ethiopia: a systematic review and meta-analysis.BMC Pharmacol Toxicol. 2019 Nov 1;20(1):63. doi: 10.1186/s40360-019-0361-3. BMC Pharmacol Toxicol. 2019. PMID: 31675986 Free PMC article.
-
Highly active antiretroviral therapy is necessary but not sufficient. A systematic review and meta-analysis of mortality incidence rates and predictors among HIV-infected adults receiving treatment in Ethiopia, a surrogate study for resource-poor settings.BMC Public Health. 2024 Jun 28;24(1):1735. doi: 10.1186/s12889-024-19268-1. BMC Public Health. 2024. PMID: 38943123 Free PMC article.
Cited by
-
Antiretroviral therapy regimen modification rates and associated factors in a cohort of HIV/AIDS patients in Asmara, Eritrea: a 16-year retrospective analysis.Sci Rep. 2023 Mar 14;13(1):4183. doi: 10.1038/s41598-023-30804-8. Sci Rep. 2023. PMID: 36918596 Free PMC article.
-
Second-line antiretroviral therapy regimen change among adults living with HIV in Amhara region: a multi-centered retrospective follow-up study.BMC Res Notes. 2019 Jul 15;12(1):407. doi: 10.1186/s13104-019-4429-3. BMC Res Notes. 2019. PMID: 31307513 Free PMC article.
-
Time to initial highly active antiretroviral therapy discontinuation and its predictors among HIV patients in Felege Hiwot comprehensive specialized hospital: a retrospective cohort study.AIDS Res Ther. 2021 Dec 4;18(1):93. doi: 10.1186/s12981-021-00418-z. AIDS Res Ther. 2021. PMID: 34863200 Free PMC article.
-
Time to lost to follow-up and its predictors among adult patients receiving antiretroviral therapy retrospective follow-up study Amhara Northwest Ethiopia.Sci Rep. 2022 Feb 21;12(1):2916. doi: 10.1038/s41598-022-07049-y. Sci Rep. 2022. PMID: 35190629 Free PMC article.
-
Pattern of and reasons for antiretroviral therapy regimen change among adult HIV/AIDS patients at regional hospital in Eastern Ethiopia: A 10-year retrospective study.SAGE Open Med. 2019 Feb 1;7:2050312119827092. doi: 10.1177/2050312119827092. eCollection 2019. SAGE Open Med. 2019. PMID: 30746143 Free PMC article.
References
-
- Jima YT. MT, ANGAMO, WABE N-T. Causes for antiretroviral regimen change among HIV/AIDS patients in Addis Ababa, Ethiopia. Tanzan J Health Res. 2013;15(1):1–9. - PubMed
-
- Ministry of Health. National Comprehensive HIV Care and Treatment Training for Health care Providers. Ethiopia; 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

