Atypical skin reaction in a patient treated with gefitinib for advanced lung cancer: A case report and review of the literature
- PMID: 26889239
- PMCID: PMC4726852
- DOI: 10.3892/etm.2015.2881
Atypical skin reaction in a patient treated with gefitinib for advanced lung cancer: A case report and review of the literature
Abstract
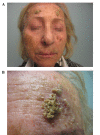
Gefitinib is a selective epidermal growth factor receptor tyrosine kinase inhibitor utilized for the treatment of advanced non-small cell lung carcinoma. The most commonly reported adverse event during gefitinib therapy is skin rash, particularly a papulopustular acne-like eruption. Cutaneous toxicities can affect treatment compliance and the quality of life of the patient. The present study reports a case of gefitinib-induced atypical skin reaction in a 73-year-old woman with advanced non-small cell lung cancer, who developed a squamous-crusted eruption on her face after 4 weeks of oral treatment with gefitinib at a dose of 250 mg/day. The patient was treated with 100 mg minocyclin (2 tablets/day, orally) and with ryfamicin topically. A complete resolution of the lesions was observed 2 weeks later. The present case report explored the pathogenesis of this skin manifestation, focusing on the underlying immunological mechanisms. A review of the literature concerning skin reactions to gefitinib was also conducted.
Keywords: epidermal growth factor receptor; gefitinib; skin immune system.
Figures

References
-
- Peréz-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulières D, et al. HER1/EGFR inhibitor-associated rash: Future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10:345–356. doi: 10.1634/theoncologist.10-5-345. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
