Variations in Practice to Therapeutic Monitoring of Tacrolimus following Primary Adult Liver Transplantation
- PMID: 26889368
- PMCID: PMC4756259
Variations in Practice to Therapeutic Monitoring of Tacrolimus following Primary Adult Liver Transplantation
Abstract
Background: There is limited clinical evidence evaluating the correlation between immunosuppressant monitoring practice and transplant outcomes.
Objective: To assess current practice of tacrolimus trough monitoring in early post-operative period following liver transplantation (LT), and its impact on outcomes.
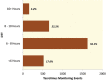
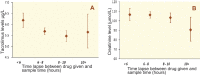
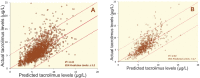
Methods: The duration to trough levels (DTT) were calculated in patients undergoing primary LT. The impact of variability in DTT on graft rejection episodes, serum tacrolimus level and renal function was assessed. These results were converted into a drug level estimation tool, which was validated in a prospective cohort of patients.
Results: 2946 events in 274 patients were evaluated. The median DTT was 7:19 hrs (range: 27 min to 19:38 hrs). In 72% (2140 events) of the occasions, DTT was <8 hrs. There was a significant (p=0.022) correlation between DTT and tacrolimus level. Despite clinical decisions were taken to modify the dose of tacrolimus based on trough level, neither did DTT affect the average creatinine levels (p=0.923), nor the variability in DTT did affect acute rejection (p=0.914, and 0.712, respectively). A dose estimation tool was developed and applied to validation cohort (n=612), and returned a moderate R(2) value of 0.50.
Conclusion: There is a significant variation in the "real world" monitoring of tacrolimus with DTT in majority of measurements falling below recommendations; reassuringly, this did not lead to adverse transplant sequelae.
Keywords: Drug monitoring; Immunosuppressive agents [Pharmacological action]; Outcome assessment (Health care); Patient outcome assessment; Tacrolimus; Transplantation.
Figures
Similar articles
-
Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients.Clin Pharmacokinet. 2004;43(11):741-62. doi: 10.2165/00003088-200443110-00005. Clin Pharmacokinet. 2004. PMID: 15301578 Clinical Trial.
-
Clinically Significant Drug Interaction Between Clotrimazole and Tacrolimus in Pancreas Transplant Recipients and Associated Risk of Allograft Rejection.Pharmacotherapy. 2016 Mar;36(3):335-41. doi: 10.1002/phar.1718. Epub 2016 Mar 11. Pharmacotherapy. 2016. PMID: 26877191
-
Performance of modified-release tacrolimus after conversion in liver transplant patients indicates potentially favorable outcomes in selected cohorts.Liver Transpl. 2015 Jan;21(1):29-37. doi: 10.1002/lt.24022. Liver Transpl. 2015. PMID: 25312292
-
Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation.Clin Pharmacokinet. 2004;43(10):623-53. doi: 10.2165/00003088-200443100-00001. Clin Pharmacokinet. 2004. PMID: 15244495 Review.
-
New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine.Clin Biochem. 2001 Feb;34(1):9-16. doi: 10.1016/s0009-9120(00)00175-2. Clin Biochem. 2001. PMID: 11239509 Review.
Cited by
-
Evaluation of Flexible Tacrolimus Drug Concentration Monitoring Approach in Patients Receiving Extended-Release Once-Daily Tacrolimus Tablets.J Clin Pharmacol. 2018 Jul;58(7):891-896. doi: 10.1002/jcph.1082. Epub 2018 Feb 20. J Clin Pharmacol. 2018. PMID: 29462506 Free PMC article. Clinical Trial.
-
Population Pharmacokinetics of Tacrolimus in Transplant Recipients: What Did We Learn About Sources of Interindividual Variabilities?J Clin Pharmacol. 2019 Mar;59(3):309-325. doi: 10.1002/jcph.1325. Epub 2018 Oct 29. J Clin Pharmacol. 2019. PMID: 30371942 Free PMC article. Review.
-
EnGraft: a multicentre, open-label, randomised, two-arm, superiority study protocol to assess bioavailability and practicability of Envarsus® versus Advagraf™ in liver transplant recipients.Trials. 2023 May 11;24(1):325. doi: 10.1186/s13063-023-07344-7. Trials. 2023. PMID: 37170284 Free PMC article.
-
High tacrolimus blood concentrations early after lung transplantation and the risk of kidney injury.Eur J Clin Pharmacol. 2017 May;73(5):573-580. doi: 10.1007/s00228-017-2204-8. Epub 2017 Jan 28. Eur J Clin Pharmacol. 2017. PMID: 28132082 Free PMC article.
-
Cost-effectiveness analysis of CYP3A5 genotype-guided tacrolimus dosing in solid organ transplantation using real-world data.Pharmacogenomics J. 2024 May 15;24(3):14. doi: 10.1038/s41397-024-00334-1. Pharmacogenomics J. 2024. PMID: 38750044
References
-
- Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group. Lancet. 1994;344:423–8. - PubMed
-
- Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 1987;40:1256–65. - PubMed
-
- Astellas. Highlights of prescribing information. [(Accessed August 3, 2014)]. Available from: http://www.astellas.us/docs/prograf.pdf.
-
- Laskow DA, Vincenti F, Neylan JF, et al. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900–905. - PubMed
LinkOut - more resources
Full Text Sources
