Decreased Time to Treatment Initiation for Multidrug-Resistant Tuberculosis Patients after Use of Xpert MTB/RIF Test, Latvia
- PMID: 26889608
- PMCID: PMC4766893
- DOI: 10.3201/eid2203.151227
Decreased Time to Treatment Initiation for Multidrug-Resistant Tuberculosis Patients after Use of Xpert MTB/RIF Test, Latvia
Abstract
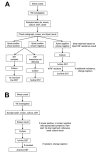
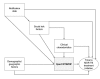
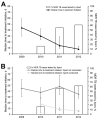
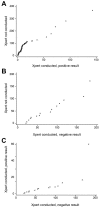
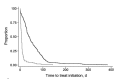
Few studies have examined whether the Xpert MTB/RIF test improves time to treatment initiation for persons with multidrug-resistant tuberculosis (MDR TB). We determined the impact of this test in Latvia, where it was introduced in 2010. After descriptive analyses of pulmonary MDR TB patients in Latvia during 2009-2012, time to treatment initiation was calculated, and univariate and multivariable accelerated failure time models were constructed. Univariate results showed strong evidence of an association between having rifampin-resistant TB detected by Xpert MTB/RIF and reduced time to treatment initiation versus the test not being used. A multivariable model stratifying by previous TB showed similar results. Our finding that in Latvia, time to treatment initiation was decreased for MDR TB cases that were rifampin-resistant TB by XpertMTB/RIF has implications for the use of this test in other settings with a high burden of MDR TB in which rifampin resistance is highly predictive of MDR TB.
Keywords: Latvia; MDR TB; Xpert MTB/RIF; antimicrobial resistance; bacteria; molecular diagnostics; multidrug resistance; multidrug-resistant tuberculosis; pulmonary; rifampin; time to treatment initiation; tuberculosis and other mycobacteria.
Figures
References
-
- World Health Organization. Global tuberculosis report, 2015. [cited 2015 Nov 2]. http://apps.who.int/iris/bitstream/10665/191102/9789241565059_eng.pdf
-
- World Health Organization. WHO endorses new rapid tuberculosis test, 2010. [cited 2015 May 18]. http://who.int/mediacentre/news/releases/2010/tb_test_20101208/en/
-
- World Health Organization. Revision of automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Policy statement, 2013. [cited 2015 May 18]. http://apps.who.int/iris/handle/10665/112472 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

