The Interplay between Myc and CTP Synthase in Drosophila
- PMID: 26889675
- PMCID: PMC4759343
- DOI: 10.1371/journal.pgen.1005867
The Interplay between Myc and CTP Synthase in Drosophila
Abstract
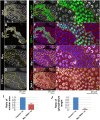
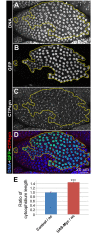
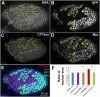
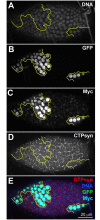
CTP synthase (CTPsyn) is essential for the biosynthesis of pyrimidine nucleotides. It has been shown that CTPsyn is incorporated into a novel cytoplasmic structure which has been termed the cytoophidium. Here, we report that Myc regulates cytoophidium formation during Drosophila oogenesis. We have found that Myc protein levels correlate with cytoophidium abundance in follicle epithelia. Reducing Myc levels results in cytoophidium loss and small nuclear size in follicle cells, while overexpression of Myc increases the length of cytoophidia and the nuclear size of follicle cells. Ectopic expression of Myc induces cytoophidium formation in late stage follicle cells. Furthermore, knock-down of CTPsyn is sufficient to suppress the overgrowth phenotype induced by Myc overexpression, suggesting CTPsyn acts downstream of Myc and is required for Myc-mediated cell size control. Taken together, our data suggest a functional link between Myc, a renowned oncogene, and the essential nucleotide biosynthetic enzyme CTPsyn.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
MicroRNA regulation of CTP synthase and cytoophidium in Drosophila melanogaster.Exp Cell Res. 2019 Dec 15;385(2):111688. doi: 10.1016/j.yexcr.2019.111688. Epub 2019 Nov 1. Exp Cell Res. 2019. PMID: 31678212
-
CTP Synthase Is Required for Optic Lobe Homeostasis in Drosophila.J Genet Genomics. 2015 May 20;42(5):261-74. doi: 10.1016/j.jgg.2015.04.006. Epub 2015 May 18. J Genet Genomics. 2015. PMID: 26059773 Free PMC article.
-
Ectopic miR-975 induces CTP synthase directed cell proliferation and differentiation in Drosophila melanogaster.Sci Rep. 2019 Apr 15;9(1):6096. doi: 10.1038/s41598-019-42369-6. Sci Rep. 2019. PMID: 30988367 Free PMC article.
-
CTP synthase: the hissing of the cellular serpent.Histochem Cell Biol. 2022 Dec;158(6):517-534. doi: 10.1007/s00418-022-02133-w. Epub 2022 Jul 26. Histochem Cell Biol. 2022. PMID: 35881195 Free PMC article. Review.
-
CTPS cytoophidia in Drosophila: distribution, regulation, and physiological roles.Exp Cell Res. 2025 Apr 15;447(2):114536. doi: 10.1016/j.yexcr.2025.114536. Epub 2025 Mar 22. Exp Cell Res. 2025. PMID: 40122502 Review.
Cited by
-
CTP synthase polymerization in germline cells of the developing Drosophila egg supports egg production.Biol Open. 2020 Jul 21;9(7):bio050328. doi: 10.1242/bio.050328. Biol Open. 2020. PMID: 32580972 Free PMC article.
-
Polyploidy in development and tumor models in Drosophila.Semin Cancer Biol. 2022 Jun;81:106-118. doi: 10.1016/j.semcancer.2021.09.011. Epub 2021 Sep 22. Semin Cancer Biol. 2022. PMID: 34562587 Free PMC article. Review.
-
High level of CTP synthase induces formation of cytoophidia in cortical neurons and impairs corticogenesis.Histochem Cell Biol. 2018 Jan;149(1):61-73. doi: 10.1007/s00418-017-1612-2. Epub 2017 Oct 3. Histochem Cell Biol. 2018. PMID: 28975414
-
Spatial Organization of Metabolic Enzyme Complexes in Cells.Biochemistry. 2017 Jun 27;56(25):3184-3196. doi: 10.1021/acs.biochem.7b00249. Epub 2017 Jun 16. Biochemistry. 2017. PMID: 28580779 Free PMC article. Review.
-
The Impact of Developmental and Metabolic Cues on Cytoophidium Formation.Int J Mol Sci. 2024 Sep 19;25(18):10058. doi: 10.3390/ijms251810058. Int J Mol Sci. 2024. PMID: 39337544 Free PMC article. Review.
References
-
- Lieberman I (1956) Enzymatic amination of uridine triphosphate to cytidine triphosphate. J Biol Chem 222: 765–775. - PubMed
-
- Long CW, Pardee AB (1967) Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem 242: 4715–4721. - PubMed
-
- Levitzki A, Koshland DE Jr., (1971) Cytidine triphosphate synthetase. Covalent intermediates and mechanisms of action. Biochemistry 10: 3365–3371. - PubMed
-
- Levitzki A, Stallcup WB, Koshland DE Jr., (1971) Half-of-the-sites reactivity and the conformational states of cytidine triphosphate synthetase. Biochemistry 10: 3371–3378. - PubMed
-
- Robertson JG (1995) Determination of subunit dissociation constants in native and inactivated CTP synthetase by sedimentation equilibrium. Biochemistry 34: 7533–7541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

