Inhibition of ice recrystallization and cryoprotective activity of wheat proteins in liver and pancreatic cells
- PMID: 26889747
- PMCID: PMC4838640
- DOI: 10.1002/pro.2903
Inhibition of ice recrystallization and cryoprotective activity of wheat proteins in liver and pancreatic cells
Abstract
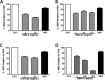
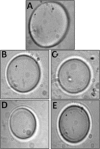
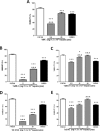
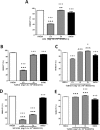
Efficient cryopreservation of cells at ultralow temperatures requires the use of substances that help maintain viability and metabolic functions post-thaw. We are developing new technology where plant proteins are used to substitute the commonly-used, but relatively toxic chemical dimethyl sulfoxide. Recombinant forms of four structurally diverse wheat proteins, TaIRI-2 (ice recrystallization inhibition), TaBAS1 (2-Cys peroxiredoxin), WCS120 (dehydrin), and TaENO (enolase) can efficiently cryopreserve hepatocytes and insulin-secreting INS832/13 cells. This study shows that TaIRI-2 and TaENO are internalized during the freeze-thaw process, while TaBAS1 and WCS120 remain at the extracellular level. Possible antifreeze activity of the four proteins was assessed. The "splat cooling" method for quantifying ice recrystallization inhibition activity (a property that characterizes antifreeze proteins) revealed that TaIRI-2 and TaENO are more potent than TaBAS1 and WCS120. Because of their ability to inhibit ice recrystallization, the wheat recombinant proteins TaIRI-2 and TaENO are promising candidates and could prove useful to improve cryopreservation protocols for hepatocytes and insulin-secreting cells, and possibly other cell types. TaENO does not have typical ice-binding domains, and the TargetFreeze tool did not predict an antifreeze capacity, suggesting the existence of nontypical antifreeze domains. The fact that TaBAS1 is an efficient cryoprotectant but does not show antifreeze activity indicates a different mechanism of action. The cryoprotective properties conferred by WCS120 depend on biochemical properties that remain to be determined. Overall, our results show that the proteins' efficiencies vary between cell types, and confirm that a combination of different protection mechanisms is needed to successfully cryopreserve mammalian cells.
Keywords: cryopreservation; ice recrystallization inhibition; mammalian cell; plant protein.
© 2016 The Protein Society.
Figures
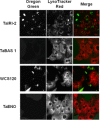
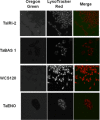
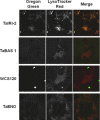
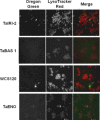

References
-
- Hughes RD, Mitry RR, Dhawan A (2012) Current status of hepatocyte transplantation. Transplantation 93:342–347. - PubMed
-
- Kabelitz D, Geissler EK, Soria B, Schroeder IS, Fandrich F, Chatenoud L (2008) Toward cell‐based therapy of type I diabetes. Trends Immunol 29:68–74. - PubMed
-
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert‐Margolis V, Bluestone J, Lakey JR (2006) International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330. - PubMed
-
- O'Brien PJ (2014) High‐content analysis in toxicology: screening substances for human toxicity potential, elucidating subcellular mechanisms and in vivo use as translational safety biomarkers. Basic Clin Pharmacol Toxicol 115:4–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

