Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin
- PMID: 26889752
- PMCID: PMC4982081
- DOI: 10.1111/nph.13882
Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin
Abstract
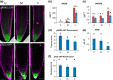
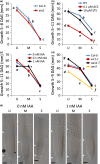
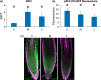
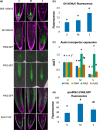
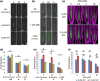
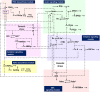
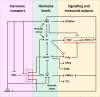
Understanding the mechanisms regulating root development under drought conditions is an important question for plant biology and world agriculture. We examine the effect of osmotic stress on abscisic acid (ABA), cytokinin and ethylene responses and how they mediate auxin transport, distribution and root growth through effects on PIN proteins. We integrate experimental data to construct hormonal crosstalk networks to formulate a systems view of root growth regulation by multiple hormones. Experimental analysis shows: that ABA-dependent and ABA-independent stress responses increase under osmotic stress, but cytokinin responses are only slightly reduced; inhibition of root growth under osmotic stress does not require ethylene signalling, but auxin can rescue root growth and meristem size; osmotic stress modulates auxin transporter levels and localization, reducing root auxin concentrations; PIN1 levels are reduced under stress in an ABA-dependent manner, overriding ethylene effects; and the interplay among ABA, ethylene, cytokinin and auxin is tissue-specific, as evidenced by differential responses of PIN1 and PIN2 to osmotic stress. Combining experimental analysis with network construction reveals that ABA regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin.
Keywords: Arabidopsis thaliana; PIN proteins; abscisic acid (ABA); auxin; ethylene; hormonal crosstalk, osmotic stress; root development.
© 2016 The Authors. New Phytologist © 2016 New Phytologist Trust.
Figures

Similar articles
-
ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis.Plant Cell. 2010 Nov;22(11):3560-73. doi: 10.1105/tpc.110.074641. Epub 2010 Nov 19. Plant Cell. 2010. PMID: 21097710 Free PMC article.
-
ABSCISIC ACID INSENSITIVE 3 promotes auxin signalling by regulating SHY2 expression to control primary root growth in response to dehydration stress.J Exp Bot. 2024 Aug 28;75(16):5111-5129. doi: 10.1093/jxb/erae237. J Exp Bot. 2024. PMID: 38770693
-
Transcription factor WRKY46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis.Plant J. 2015 Oct;84(1):56-69. doi: 10.1111/tpj.12958. Epub 2015 Sep 18. Plant J. 2015. PMID: 26252246
-
Crosstalk Complexities between Auxin, Cytokinin, and Ethylene in Arabidopsis Root Development: From Experiments to Systems Modeling, and Back Again.Mol Plant. 2017 Dec 4;10(12):1480-1496. doi: 10.1016/j.molp.2017.11.002. Epub 2017 Nov 21. Mol Plant. 2017. PMID: 29162416 Review.
-
Deciphering Auxin-Ethylene Crosstalk at a Systems Level.Int J Mol Sci. 2018 Dec 14;19(12):4060. doi: 10.3390/ijms19124060. Int J Mol Sci. 2018. PMID: 30558241 Free PMC article. Review.
Cited by
-
Primary multistep phosphorelay activation comprises both cytokinin and abiotic stress responses: insights from comparative analysis of Brassica type-A response regulators.J Exp Bot. 2024 Oct 30;75(20):6346-6368. doi: 10.1093/jxb/erae335. J Exp Bot. 2024. PMID: 39171371 Free PMC article.
-
Coping With Water Shortage: An Update on the Role of K+, Cl-, and Water Membrane Transport Mechanisms on Drought Resistance.Front Plant Sci. 2019 Dec 20;10:1619. doi: 10.3389/fpls.2019.01619. eCollection 2019. Front Plant Sci. 2019. PMID: 31921262 Free PMC article. Review.
-
Formation and Development of Taproots in Deciduous Tree Species.Front Plant Sci. 2021 Dec 2;12:772567. doi: 10.3389/fpls.2021.772567. eCollection 2021. Front Plant Sci. 2021. PMID: 34925417 Free PMC article. Review.
-
An artificial intelligence-integrated analysis of the effect of drought stress on root traits of "modern" and "ancient" wheat varieties.Front Plant Sci. 2023 Oct 13;14:1241281. doi: 10.3389/fpls.2023.1241281. eCollection 2023. Front Plant Sci. 2023. PMID: 37900753 Free PMC article.
-
The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways.Front Plant Sci. 2017 Aug 25;8:1493. doi: 10.3389/fpls.2017.01493. eCollection 2017. Front Plant Sci. 2017. PMID: 28890725 Free PMC article.
References
-
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van der Straeten D, Peng JR, Harberd NP. 2006. Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94. - PubMed
-
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GTS, Genschik P. 2009. Gibberellin signaling controls cell proliferation rate in Arabidopsis . Current Biology 19: 1188–1193. - PubMed
-
- Alam SM. 1999. Nutrient uptake by plants under stress conditions. Handbook of Plant and Crop Stress 2: 285–313.
-
- Anderson LE. 1954. Hoyer's solution as a rapid permanent mounting medium for bryophytes. Bryologist 57: 242–244.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

