Molecular mechanisms of biological aging in intervertebral discs
- PMID: 26890203
- PMCID: PMC4988945
- DOI: 10.1002/jor.23195
Molecular mechanisms of biological aging in intervertebral discs
Abstract
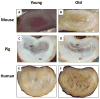
Advanced age is the greatest risk factor for the majority of human ailments, including spine-related chronic disability and back pain, which stem from age-associated intervertebral disc degeneration (IDD). Given the rapid global rise in the aging population, understanding the biology of intervertebral disc aging in order to develop effective therapeutic interventions to combat the adverse effects of aging on disc health is now imperative. Fortunately, recent advances in aging research have begun to shed light on the basic biological process of aging. Here we review some of these insights and organize the complex process of disc aging into three different phases to guide research efforts to understand the biology of disc aging. The objective of this review is to provide an overview of the current knowledge and the recent progress made to elucidate specific molecular mechanisms underlying disc aging. In particular, studies over the last few years have uncovered cellular senescence and genomic instability as important drivers of disc aging. Supporting evidence comes from DNA repair-deficient animal models that show increased disc cellular senescence and accelerated disc aging. Additionally, stress-induced senescent cells have now been well documented to secrete catabolic factors, which can negatively impact the physiology of neighboring cells and ECM. These along with other molecular drivers of aging are reviewed in depth to shed crucial insights into the underlying mechanisms of age-related disc degeneration. We also highlight molecular targets for novel therapies and emerging candidate therapeutics that may mitigate age-associated IDD. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:1289-1306, 2016.
Keywords: aging; cellular senescence; inflammation; intervertebral disc; oxidative damage.
© 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures


References
-
- World Population Prospects The 2015 Revision. at < http://esa.un.org/unpd/wpp/Publications/Files/Key_Findings_WPP_2015.pdf>.
-
- The Burden of Musculoskeletal Diseases in the United States. American Academy of Orthopaedic Surgeons; 2008. at < http://www.boneandjointburden.org/docs/TheBurden of Musculoskeletal Dise...>.
-
- Derby R, Lee SH, Kim BJ. Discography. In: Slipman CW, Derby R, Simeone FA, Mayer TG , editors. Interventional Spine: an algorithmic approach. Elsevier; 2008. pp. 291–302.
-
- Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey. 1991–1994 at < http://www.jrheum.org/content/33/11/2271.full.pdf>. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

