Direct Acylation of C(sp(3))-H Bonds Enabled by Nickel and Photoredox Catalysis
- PMID: 26890705
- PMCID: PMC4807873
- DOI: 10.1002/anie.201511438
Direct Acylation of C(sp(3))-H Bonds Enabled by Nickel and Photoredox Catalysis
Abstract
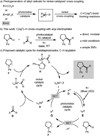
Using nickel and photoredox catalysis, the direct functionalization of C(sp(3))-H bonds of N-aryl amines by acyl electrophiles is described. The method affords a diverse range of α-amino ketones at room temperature and is amenable to late-stage coupling of complex and biologically relevant groups. C(sp(3))-H activation occurs by photoredox-mediated oxidation to generate α-amino radicals which are intercepted by nickel in catalytic C(sp(3))-C coupling. The merger of these two modes of catalysis leverages nickel's unique properties in alkyl cross-coupling while avoiding limitations commonly associated with transition-metal-mediated C(sp(3))-H activation, including requirements for chelating directing groups and high reaction temperatures.
Keywords: C−H activation; acylation; cross-coupling; nickel; photochemistry.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp(3)-sp(2) Cross-Coupling.Acc Chem Res. 2016 Jul 19;49(7):1429-39. doi: 10.1021/acs.accounts.6b00214. Epub 2016 Jul 5. Acc Chem Res. 2016. PMID: 27379472 Free PMC article.
-
Direct Enantioselective C(sp3)-H Acylation for the Synthesis of α-Amino Ketones.J Am Chem Soc. 2020 Nov 11;142(45):19058-19064. doi: 10.1021/jacs.0c10471. Epub 2020 Oct 30. J Am Chem Soc. 2020. PMID: 33125845
-
Ni-Catalyzed C-C Couplings Using Alkyl Electrophiles.Top Curr Chem (Cham). 2016 Oct;374(5):66. doi: 10.1007/s41061-016-0067-6. Epub 2016 Aug 31. Top Curr Chem (Cham). 2016. PMID: 27580894 Review.
-
Photoredox catalysis in nickel-catalyzed C-H functionalization.Beilstein J Org Chem. 2021 Aug 31;17:2209-2259. doi: 10.3762/bjoc.17.143. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34621388 Free PMC article. Review.
Cited by
-
Site- and enantioselective cross-coupling of saturated N-heterocycles with carboxylic acids by cooperative Ni/photoredox catalysis.Nat Commun. 2023 Jan 9;14(1):125. doi: 10.1038/s41467-023-35800-0. Nat Commun. 2023. PMID: 36624097 Free PMC article.
-
Ketones from Nickel-Catalyzed Decarboxylative, Non-Symmetric Cross-Electrophile Coupling of Carboxylic Acid Esters.Angew Chem Int Ed Engl. 2019 Aug 26;58(35):12081-12085. doi: 10.1002/anie.201906000. Epub 2019 Jul 30. Angew Chem Int Ed Engl. 2019. PMID: 31287943 Free PMC article.
-
Alcohols as Latent Coupling Fragments for Metallaphotoredox Catalysis: sp3-sp2 Cross-Coupling of Oxalates with Aryl Halides.J Am Chem Soc. 2016 Oct 26;138(42):13862-13865. doi: 10.1021/jacs.6b09533. Epub 2016 Oct 17. J Am Chem Soc. 2016. PMID: 27718570 Free PMC article.
-
Nickel-Catalyzed Synthesis of Dialkyl Ketones from the Coupling of N-Alkyl Pyridinium Salts with Activated Carboxylic Acids.Angew Chem Int Ed Engl. 2020 Aug 3;59(32):13484-13489. doi: 10.1002/anie.202002271. Epub 2020 Jun 5. Angew Chem Int Ed Engl. 2020. PMID: 32374951 Free PMC article.
-
Radical C(sp3)-H functionalization and cross-coupling reactions.Nat Rev Chem. 2022 Jun;6(6):405-427. doi: 10.1038/s41570-022-00388-4. Epub 2022 May 17. Nat Rev Chem. 2022. PMID: 35965690 Free PMC article.
References
-
- Kakiuchi F, Kochi T. Synthesis. 2008:3013–3039.
- Sehnal P, Taylor RJK, Fairlamb IJS. Chem. Rev. 2010;110:824–889. - PubMed
- Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. Int. Ed. 2012;51:8960–9009. Angew. Chem. 2012, 124, 9092–9142. - PubMed
- Chen DY-K, Youn SW. Chem. Eur. J. 2012;18:9452–9474. - PubMed
- Wencel-Delord J, Glorius F. Nat. Chem. 2013;5:369–375. - PubMed
-
- Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654–2672. - PubMed
- Bellina F, Rossi R. Chem. Rev. 2010;110:1082–1146. - PubMed
- McMurray L, O’Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885–1898. - PubMed
- Baudoin O. Chem. Soc. Rev. 2011;40:4902–4911. - PubMed
- Li B-J, Shi Z-J. Chem. Soc. Rev. 2012;41:5588–5598. - PubMed
- Rouquet G, Chatani N. Angew. Chem. Int. Ed. 2013;52:11726–11743. Angew. Chem. 2013, 125, 11942–11959. - PubMed
-
- Tellis JC, Primer DN, Molander GA. Science. 2014;345:433–436. - PMC - PubMed
- Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437–440. - PMC - PubMed
- Noble A, McCarver SJ, MacMillan DWC. J. Am. Chem. Soc. 2015;137:624–627. - PMC - PubMed
- Chu L, Lipshultz JM, MacMillan DWC. Angew. Chem. Int. Ed. 2015;54:7929–7933. Angew. Chem. 2015, 127, 8040–8044. - PMC - PubMed
- Primer DN, Karakaya I, Tellis JC, Molander GA. J. Am. Chem. Soc. 2015;137:2195–2198. - PMC - PubMed
- Karakaya I, Primer DN, Molander GA. Org. Lett. 2015;17:3294–3297. - PMC - PubMed
- Le C, MacMillan DWC. J. Am. Chem. Soc. 2015;137:11938–11941. - PMC - PubMed
-
- Tucker JW, Stephenson CRJ. J. Org. Chem. 2012;77:1617–1622. - PubMed
- Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. - PMC - PubMed
- Yoon TP, Ischay MA, Du J. Nat. Chem. 2010;2:527–532. - PubMed
- Xuan J, Xiao W-J. Angew. Chem. Int. Ed. 2012;51:6828–6838. Angew. Chem. 2012, 124, 6934–6944. - PubMed
- Fagnoni M, Dondi D, Ravelli D, Albini A. Chem. Rev. 2007;107:2725–2756. - PubMed
- Xi Y, Yi H, Lei A. Org. Biomol. Chem. 2013;11:2387–2403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources