Direct Acylation of C(sp(3))-H Bonds Enabled by Nickel and Photoredox Catalysis
- PMID: 26890705
- PMCID: PMC4807873
- DOI: 10.1002/anie.201511438
Direct Acylation of C(sp(3))-H Bonds Enabled by Nickel and Photoredox Catalysis
Abstract
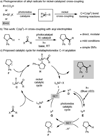
Using nickel and photoredox catalysis, the direct functionalization of C(sp(3))-H bonds of N-aryl amines by acyl electrophiles is described. The method affords a diverse range of α-amino ketones at room temperature and is amenable to late-stage coupling of complex and biologically relevant groups. C(sp(3))-H activation occurs by photoredox-mediated oxidation to generate α-amino radicals which are intercepted by nickel in catalytic C(sp(3))-C coupling. The merger of these two modes of catalysis leverages nickel's unique properties in alkyl cross-coupling while avoiding limitations commonly associated with transition-metal-mediated C(sp(3))-H activation, including requirements for chelating directing groups and high reaction temperatures.
Keywords: C−H activation; acylation; cross-coupling; nickel; photochemistry.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Kakiuchi F, Kochi T. Synthesis. 2008:3013–3039.
- Sehnal P, Taylor RJK, Fairlamb IJS. Chem. Rev. 2010;110:824–889. - PubMed
- Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. Int. Ed. 2012;51:8960–9009. Angew. Chem. 2012, 124, 9092–9142. - PubMed
- Chen DY-K, Youn SW. Chem. Eur. J. 2012;18:9452–9474. - PubMed
- Wencel-Delord J, Glorius F. Nat. Chem. 2013;5:369–375. - PubMed
-
- Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654–2672. - PubMed
- Bellina F, Rossi R. Chem. Rev. 2010;110:1082–1146. - PubMed
- McMurray L, O’Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885–1898. - PubMed
- Baudoin O. Chem. Soc. Rev. 2011;40:4902–4911. - PubMed
- Li B-J, Shi Z-J. Chem. Soc. Rev. 2012;41:5588–5598. - PubMed
- Rouquet G, Chatani N. Angew. Chem. Int. Ed. 2013;52:11726–11743. Angew. Chem. 2013, 125, 11942–11959. - PubMed
-
- Tellis JC, Primer DN, Molander GA. Science. 2014;345:433–436. - PMC - PubMed
- Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437–440. - PMC - PubMed
- Noble A, McCarver SJ, MacMillan DWC. J. Am. Chem. Soc. 2015;137:624–627. - PMC - PubMed
- Chu L, Lipshultz JM, MacMillan DWC. Angew. Chem. Int. Ed. 2015;54:7929–7933. Angew. Chem. 2015, 127, 8040–8044. - PMC - PubMed
- Primer DN, Karakaya I, Tellis JC, Molander GA. J. Am. Chem. Soc. 2015;137:2195–2198. - PMC - PubMed
- Karakaya I, Primer DN, Molander GA. Org. Lett. 2015;17:3294–3297. - PMC - PubMed
- Le C, MacMillan DWC. J. Am. Chem. Soc. 2015;137:11938–11941. - PMC - PubMed
-
- Tucker JW, Stephenson CRJ. J. Org. Chem. 2012;77:1617–1622. - PubMed
- Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. - PMC - PubMed
- Yoon TP, Ischay MA, Du J. Nat. Chem. 2010;2:527–532. - PubMed
- Xuan J, Xiao W-J. Angew. Chem. Int. Ed. 2012;51:6828–6838. Angew. Chem. 2012, 124, 6934–6944. - PubMed
- Fagnoni M, Dondi D, Ravelli D, Albini A. Chem. Rev. 2007;107:2725–2756. - PubMed
- Xi Y, Yi H, Lei A. Org. Biomol. Chem. 2013;11:2387–2403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources