Innate lymphoid cells in asthma: Will they take your breath away?
- PMID: 26891006
- PMCID: PMC4894653
- DOI: 10.1002/eji.201444557
Innate lymphoid cells in asthma: Will they take your breath away?
Abstract
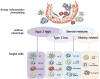
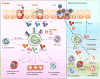
Asthma is a complex and heterogeneous disease that is characterized by airway hyper-reactivity (AHR) and airway inflammation. Although asthma was long thought to be driven by allergen-reactive TH 2 cells, it has recently become clear that the pathogenesis of asthma is more complicated and associated with multiple pathways and cell types. A very exciting recent development was the discovery of innate lymphoid cells (ILCs) as key players in the pathogenesis of asthma. ILCs do not express antigen receptors but react promptly to "danger signals" from inflamed tissue and produce an array of cytokines that direct the ensuing immune response. The roles of ILCs may differ in distinct asthma phenotypes. ILC2s may be critical for initiation of adaptive immune responses in inhaled allergen-driven AHR, but may also function independently of adaptive immunity, mediating influenza-induced AHR. ILC2s also contribute to resolution of lung inflammation through their production of amphiregulin. Obesity-induced asthma is associated with expansion of IL-17A-producing ILC3s in the lungs. Furthermore, ILCs may also contribute to steroid-resistant asthma. Although the precise roles of ILCs in different types of asthma are still under investigation, it is clear that inhibition of ILC function represents a potential target that could provide novel treatments for asthma.
Keywords: Airway hyper-reactivity; Allergy; Asthma; Influenza; Innate lymphoid cells; Obesity.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
DTU is an employee of Genentech. The other authors declare no financial or commercial conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

