Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management
- PMID: 26891349
- PMCID: PMC4758728
- DOI: 10.1371/journal.ppat.1005434
Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management
Abstract
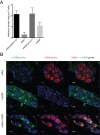
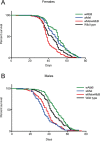
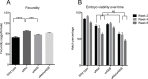
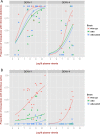
Wolbachia pipientis is an endosymbiotic bacterium estimated to chronically infect between 40-75% of all arthropod species. Aedes aegypti, the principle mosquito vector of dengue virus (DENV), is not a natural host of Wolbachia. The transinfection of Wolbachia strains such as wAlbB, wMel and wMelPop-CLA into Ae. aegypti has been shown to significantly reduce the vector competence of this mosquito for a range of human pathogens in the laboratory. This has led to wMel-transinfected Ae. aegypti currently being released in five countries to evaluate its effectiveness to control dengue disease in human populations. Here we describe the generation of a superinfected Ae. aegypti mosquito line simultaneously infected with two avirulent Wolbachia strains, wMel and wAlbB. The line carries a high overall Wolbachia density and tissue localisation of the individual strains is very similar to each respective single infected parental line. The superinfected line induces unidirectional cytoplasmic incompatibility (CI) when crossed to each single infected parental line, suggesting that the superinfection would have the capacity to replace either of the single constituent infections already present in a mosquito population. No significant differences in fitness parameters were observed between the superinfected line and the parental lines under the experimental conditions tested. Finally, the superinfected line blocks DENV replication more efficiently than the single wMel strain when challenged with blood meals from viremic dengue patients. These results suggest that the deployment of superinfections could be used to replace single infections and may represent an effective strategy to help manage potential resistance by DENV to field deployments of single infected strains.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
Renewables need a grand-challenge strategy.Nature. 2016 Oct 6;538(7623):27-29. doi: 10.1038/538030a. Nature. 2016. PMID: 27708325 No abstract available.
References
-
- Werren JH, O'Neill SL. The evolution of heritable symbionts In: O'Neill SL, Hoffmann AA, Werren JH, editors. Influential Passengers. New York: Oxford University Press; 1997. p. 1–41.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

