A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy
- PMID: 26891420
- PMCID: PMC5071720
- DOI: 10.1002/ijc.30049
A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy
Abstract
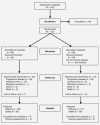
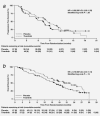
Cetuximab in combination with an irinotecan-containing regimen is a standard treatment in patients with KRAS wild-type (KRAS WT), metastatic colorectal cancer (mCRC). We investigated the addition of the oral MET inhibitor tivantinib to cetuximab + irinotecan (CETIRI) based on preclinical evidence that activation of the MET pathway may confer resistance to anti-EGFR therapy. Previously treated patients with KRAS WT advanced or mCRC were enrolled. The phase 1, open-label 3 + 3, dose-escalation study evaluated the safety and maximally tolerated dose of tivantinib plus CETIRI. The phase 2, randomized, double-blinded, placebo-controlled study of biweekly CETIRI plus tivantinib or placebo was restricted to patients who had received only one prior line of chemotherapy. The phase 2 primary endpoint was progression-free survival (PFS). The recommended phase 2 dose was tivantinib (360 mg/m(2) twice daily) with biweekly cetuximab (500 mg/m(2)) and irinotecan (180 mg/m(2)). Among 117 patients evaluable for phase 2 analysis, no statistically significant PFS difference was observed: 8.3 months on tivantinib vs. 7.3 months on placebo (HR, 0.85; 95% confidence interval, 0.55-1.33; P = 0.38). Subgroup analyses trended in favor of tivantinib in patients with MET-High tumors by immunohistochemistry, PTEN-Low tumors, or those pretreated with oxaliplatin, but subgroups were too small to draw conclusions. Neutropenia, diarrhea, nausea and rash were the most frequent severe adverse events in tivantinib-treated patients. The combination of tivantinib and CETIRI was well tolerated but did not significantly improve PFS in previously treated KRAS WT mCRC. Tivantinib may be more active in specific subgroups.
Keywords: KRAS wild-type; MET inhibitor; irinotecan; metastatic colorectal cancer; tivantinib.
© 2016 The Authors International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures
References
-
- Van Cutsem E, Nordlinger B, Cervantes A, et al. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol: 2010;21(suppl5):v93–7. - PubMed
-
- National Cancer Institute . Surveillance, Epidemiology, and End Results Stat Fact sheets: Colon and Rectum Cancer. http://seer.cancer.gov/statfacts/html/colorect.html.
-
- Benson AB, III , Bekaii‐Saab T, Chan E, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013; 11:141–52. quiz 52. - PubMed
-
- Pfeiffer P, Nielsen D, Bjerregaard J, et al. Biweekly cetuximab and irinotecan as third‐line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5‐fluorouracil. Ann Oncol 2008; 19:1141–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

