Structural determinants of muscle thin filament cooperativity
- PMID: 26891592
- PMCID: PMC4792785
- DOI: 10.1016/j.abb.2016.02.016
Structural determinants of muscle thin filament cooperativity
Abstract
End-to-end connections between adjacent tropomyosin molecules along the muscle thin filament allow long-range conformational rearrangement of the multicomponent filament structure. This process is influenced by Ca(2+) and the troponin regulatory complexes, as well as by myosin crossbridge heads that bind to and activate the filament. Access of myosin crossbridges onto actin is gated by tropomyosin, and in the case of striated muscle filaments, troponin acts as a gatekeeper. The resulting tropomyosin-troponin-myosin on-off switching mechanism that controls muscle contractility is a complex cooperative and dynamic system with highly nonlinear behavior. Here, we review key information that leads us to view tropomyosin as central to the communication pathway that coordinates the multifaceted effectors that modulate and tune striated muscle contraction. We posit that an understanding of this communication pathway provides a framework for more in-depth mechanistic characterization of myopathy-associated mutational perturbations currently under investigation by many research groups.
Keywords: Actin; Cooperativity; Myosin; Thin filament; Tropomyosin; Troponin.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
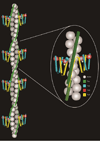



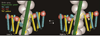
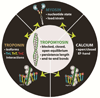
References
-
- Haselgrove JC. X-ray evidence for a conformational change in actin-containing filaments of vertebrate striated muscle. Cold Spring Harbor Symp. Quant. Biol. 1972;37:341–352.
-
- Huxley HE. Structural changes in actin and myosin-containing filaments during contraction. Cold Spring Harbor Symp. Quant. Biol. 1972;37:361–376.
-
- Parry DAD, Squire JM. Structural role of tropomyosin in muscle regulation: Analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J. Mol. Biol. 1973;75:33–55. - PubMed
-
- Lehman W, Craig R, Vibert P. Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994;368:65–67. - PubMed
-
- Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J. Mol. Biol. 1997;266:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

