Differential effects of gram-positive and gram-negative bacterial products on morphine induced inhibition of phagocytosis
- PMID: 26891899
- PMCID: PMC4759540
- DOI: 10.1038/srep21094
Differential effects of gram-positive and gram-negative bacterial products on morphine induced inhibition of phagocytosis
Erratum in
-
Erratum: Differential effects of gram-positive and gram-negative bacterial products on morphine induced inhibition of phagocytosis.Sci Rep. 2016 Apr 20;6:24011. doi: 10.1038/srep24011. Sci Rep. 2016. PMID: 27096260 Free PMC article. No abstract available.
Abstract
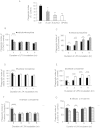
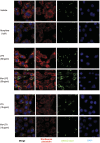
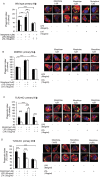
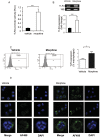
Opioid drug abusers have a greater susceptibility to gram positive (Gram (+)) bacterial infections. However, the mechanism underlying opioid modulation of Gram (+) versus Gram (-) bacterial clearance has not been investigated. In this study, we show that opioid treatment resulted in reduced phagocytosis of Gram (+), when compared to Gram (-) bacteria. We further established that LPS priming of chronic morphine treated macrophages leads to potentiated phagocytosis and killing of both Gram (+) and Gram (-) bacteria in a P-38 MAP kinase dependent signaling pathway. In contrast, LTA priming lead to inhibition of both phagocytosis and bacterial killing. This study demonstrates for the first time the differential effects of TLR4 and TLR2 agonists on morphine induced inhibition of phagocytosis. Our results suggest that the incidence and severity of secondary infections with Gram (+) bacteria would be higher in opioid abusers.
Figures




References
-
- Aderem A. & Underhill D. M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17, 593–623 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

