Modelling semi-attributable toxicity in dual-agent phase I trials with non-concurrent drug administration
- PMID: 26891942
- PMCID: PMC5157785
- DOI: 10.1002/sim.6912
Modelling semi-attributable toxicity in dual-agent phase I trials with non-concurrent drug administration
Abstract
In oncology, combinations of drugs are often used to improve treatment efficacy and/or reduce harmful side effects. Dual-agent phase I clinical trials assess drug safety and aim to discover a maximum tolerated dose combination via dose-escalation; cohorts of patients are given set doses of both drugs and monitored to see if toxic reactions occur. Dose-escalation decisions for subsequent cohorts are based on the number and severity of observed toxic reactions, and an escalation rule. In a combination trial, drugs may be administered concurrently or non-concurrently over a treatment cycle. For two drugs given non-concurrently with overlapping toxicities, toxicities occurring after administration of the first drug yet before administration of the second may be attributed directly to the first drug, whereas toxicities occurring after both drugs have been given some present ambiguity; toxicities may be attributable to the first drug only, the second drug only or the synergistic combination of both. We call this mixture of attributable and non-attributable toxicity semi-attributable toxicity. Most published methods assume drugs are given concurrently, which may not be reflective of trials with non-concurrent drug administration. We incorporate semi-attributable toxicity into Bayesian modelling for dual-agent phase I trials with non-concurrent drug administration and compare the operating characteristics to an approach where this detail is not considered. Simulations based on a trial for non-concurrent administration of intravesical Cabazitaxel and Cisplatin in early-stage bladder cancer patients are presented for several scenarios and show that including semi-attributable toxicity data reduces the number of patients given overly toxic combinations. © 2016 The Authors. Statistics in Medicine Published by John Wiley & Sons Ltd.
Keywords: Bayesian methods; adaptive designs; dose-toxicity modelling; drug combinations; phase I trials.
© 2016 The Authors. Statistics in Medicine Published by John Wiley & Sons Ltd.
Figures
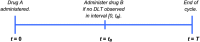



References
-
- Babb JS, Rogatko A. 2004. Bayesian methods for phase I cancer clinical trials In Advances in Clinical Trial Biostatistics, Geller NL. (ed.). Chapman & Hall/CRC Biostatistics Series: New York; 1–40.
-
- NCI . Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS, National Cancer Institute, 2009. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/ [Accessed on 8 January 2016].
-
- Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nature Reviews Drug Discovery. 2006; 5(8):649–59. - PubMed
-
- Citron M, Berry D, Cirrincione C, Hudis C, Winer E, Gradishar W, Davidson N, Martino S, Livingston R, Ingle J, Perez E, Carpenter J, Hurd D, Holland J, Smith B, Sartor C, Leung E, Abrams J, Schilsky R, Muss H, Norton L. Randomized trial of dose‐dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node‐positive primary breast cancer: first report of intergroup trial C9741/cancer and leukemia. Journal of Clinical Oncology. 2003; 21(8):1431–1439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
