Carboxylesterases: General detoxifying enzymes
- PMID: 26892220
- PMCID: PMC4985501
- DOI: 10.1016/j.cbi.2016.02.011
Carboxylesterases: General detoxifying enzymes
Abstract
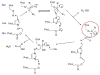
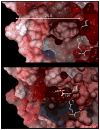
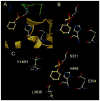
Carboxylesterases (CE) are members of the esterase family of enzymes, and as their name suggests, they are responsible for the hydrolysis of carboxylesters into the corresponding alcohol and carboxylic acid. To date, no endogenous CE substrates have been identified and as such, these proteins are thought to act as a mechanism to detoxify ester-containing xenobiotics. As a consequence, they are expressed in tissues that might be exposed to such agents (lung and gut epithelia, liver, kidney, etc.). CEs demonstrate very broad substrate specificities and can hydrolyze compounds as diverse as cocaine, oseltamivir (Tamiflu), permethrin and irinotecan. In addition, these enzymes are irreversibly inhibited by organophosphates such as Sarin and Tabun. In this overview, we will compare and contrast the two human enzymes that have been characterized, and evaluate the biology of the interaction of these proteins with organophosphates (principally nerve agents).
Keywords: Carboxylesterase; Expression; Hydrolysis; Organophosphorus compounds; Structure.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures
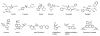

References
-
- Loevenhart AS. Further observations on the action of lipase. Am J Physiol. 1906;15:27.
-
- Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to function. Annu Rev Pharmacol Toxicol. 1998;38:257–288. - PubMed
-
- Bencharit S, Morton CL, Howard-Williams EL, Danks MK, Potter PM, Redinbo MR. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat Struct Biol. 2002;9:337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

