Calpain inhibition rescues troponin T3 fragmentation, increases Cav1.1, and enhances skeletal muscle force in aging sedentary mice
- PMID: 26892246
- PMCID: PMC4854922
- DOI: 10.1111/acel.12453
Calpain inhibition rescues troponin T3 fragmentation, increases Cav1.1, and enhances skeletal muscle force in aging sedentary mice
Abstract
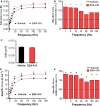
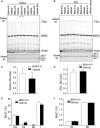
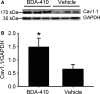
Loss of strength in human and animal models of aging can be partially attributed to a well-recognized decrease in muscle mass; however, starting at middle-age, the normalized force (force/muscle cross-sectional area) in the knee extensors and single muscle fibers declines in a curvilinear manner. Strength is lost faster than muscle mass and is a more consistent risk factor for disability and death. Reduced expression of the voltage sensor Ca(2+) channel α1 subunit (Cav1.1) with aging leads to excitation-contraction uncoupling, which accounts for a significant fraction of the decrease in skeletal muscle function. We recently reported that in addition to its classical cytoplasmic location, fast skeletal muscle troponin T3 (TnT3) is fragmented in aging mice, and both full-length TnT3 (FL-TnT3) and its carboxyl-terminal (CT-TnT3) fragment shuttle to the nucleus. Here, we demonstrate that it regulates transcription of Cacna1s, the gene encoding Cav1.1. Knocking down TnT3 in vivo downregulated Cav1.1. TnT3 downregulation or overexpression decreased or increased, respectively, Cacna1s promoter activity, and the effect was ablated by truncating the TnT3 nuclear localization sequence. Further, we mapped the Cacna1s promoter region and established the consensus sequence for TnT3 binding to Cacna1s promoter. Systemic administration of BDA-410, a specific calpain inhibitor, prevented TnT3 fragmentation, and Cacna1s and Cav1.1 downregulation and improved muscle force generation in sedentary old mice.
Keywords: aging; calcium channel; calpain; excitation-contraction coupling; skeletal muscle; troponin T.
© 2016 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



Similar articles
-
Troponin T3 associates with DNA consensus sequence that overlaps with p53 binding motifs.Exp Gerontol. 2018 Jul 15;108:35-40. doi: 10.1016/j.exger.2018.03.012. Epub 2018 Mar 27. Exp Gerontol. 2018. PMID: 29596868 Free PMC article.
-
Troponin T3 regulates nuclear localization of the calcium channel Cavβ1a subunit in skeletal muscle.Exp Cell Res. 2015 Aug 15;336(2):276-86. doi: 10.1016/j.yexcr.2015.05.005. Epub 2015 May 15. Exp Cell Res. 2015. PMID: 25981458 Free PMC article.
-
Troponin T3 ameliorates sepsis-induced diaphragm dysfunction in rats through modulation of Cacna1s.Biochem Biophys Res Commun. 2025 Aug 15;775:152112. doi: 10.1016/j.bbrc.2025.152112. Epub 2025 May 28. Biochem Biophys Res Commun. 2025. PMID: 40460485
-
Skeletal muscle CaV1.1 channelopathies.Pflugers Arch. 2020 Jul;472(7):739-754. doi: 10.1007/s00424-020-02368-3. Epub 2020 Mar 28. Pflugers Arch. 2020. PMID: 32222817 Free PMC article. Review.
-
Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle.Curr Aging Sci. 2011 Dec;4(3):248-59. doi: 10.2174/1874609811104030248. Curr Aging Sci. 2011. PMID: 21529320 Free PMC article. Review.
Cited by
-
Troponin T3 associates with DNA consensus sequence that overlaps with p53 binding motifs.Exp Gerontol. 2018 Jul 15;108:35-40. doi: 10.1016/j.exger.2018.03.012. Epub 2018 Mar 27. Exp Gerontol. 2018. PMID: 29596868 Free PMC article.
-
Effects of aging on calcium channels in skeletal muscle.Front Mol Biosci. 2025 Mar 19;12:1558456. doi: 10.3389/fmolb.2025.1558456. eCollection 2025. Front Mol Biosci. 2025. PMID: 40177518 Free PMC article. Review.
-
Commentary: Epigenetic Regulation of Phosphodiesterases 2A and 3A Underlies Compromised β-Adrenergic Signaling in an iPSC Model of Dilated Cardiomyopathy.Front Physiol. 2016 Sep 23;7:418. doi: 10.3389/fphys.2016.00418. eCollection 2016. Front Physiol. 2016. PMID: 27721795 Free PMC article. No abstract available.
-
The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability.Acta Physiol (Oxf). 2019 Mar;225(3):e13195. doi: 10.1111/apha.13195. Epub 2018 Oct 22. Acta Physiol (Oxf). 2019. PMID: 30269419 Free PMC article.
-
Implications of the complex biology and micro-environment of cardiac sarcomeres in the use of high affinity troponin antibodies as serum biomarkers for cardiac disorders.J Mol Cell Cardiol. 2020 Jun;143:145-158. doi: 10.1016/j.yjmcc.2020.05.010. Epub 2020 May 19. J Mol Cell Cardiol. 2020. PMID: 32442660 Free PMC article. Review.
References
-
- Alley DE, Koster A, Mackey D, Cawthon P, Ferrucci L, Simonsick EM, Yu B, Hardy S, Goodpaster B, Sarkisian C, Houston DK, Kritchevsky SB, Cummings S, Lee JS, Tylavsky FA, Newman A, Harris T (2010) Hospitalization and change in body composition and strength in a population‐based cohort of older persons. J. Am. Geriatr. Soc. 58, 2085–2091. - PMC - PubMed
-
- Asumda FZ, Chase PB (2012) Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation 83, 106–115. - PubMed
-
- Campbell RL, Davies PL (2012) Structure‐function relationships in calpains. Biochem. J. 447, 335–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

