Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial
- PMID: 26892612
- PMCID: PMC4777599
- DOI: 10.3892/ijo.2016.3386
Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial
Abstract
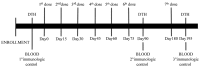
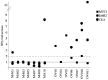
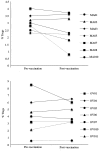
Vaccination with priming and expansion of tumour reacting T cells is an important therapeutic option to be used in combination with novel checkpoint inhibitors to increase the specificity of the T cell infiltrate and the efficacy of the treatment. In this phase I/II study, 14 high-risk disease-free ovarian (OC) and breast cancer (BC) patients after completion of standard therapies were vaccinated with MUC1, ErbB2 and carcinoembryonic antigen (CEA) HLA-A2+-restricted peptides and Montanide. Patients were subjected to 6 doses of vaccine every two weeks and a recall dose after 3 months. ECOG grade 2 toxicity was observed at the injection site. Eight out of 14 patients showed specific CD8+ T cells to at least one antigen. None of 4 patients vaccinated for compassionate use showed a CD8 activation. An OC patient who suffered from a lymph nodal recurrence, showed specific anti-ErbB2 CD8+ T cells in the bulky aortic lymph nodes suggesting homing of the activated T cells. Results confirm that peptide vaccination strategy is feasible, safe and well tolerated. In particular OC patients appear to show a higher response rate compared to BC patients. Vaccination generates a long-lasting immune response, which is strongly enhanced by recall administrations. The clinical outcome of patients enrolled in the trial appears favourable, having registered no deceased patients with a minimum follow-up of 8 years. These promising data, in line with the results of similar studies, the high compliance of patients observed and the favourable toxicity profile, support future trials of peptide vaccination in clinically disease-free patients who have completed standard treatments.
Figures

References
-
- Chan S, Friedrichs K, Noel D, Pintér T, Van Belle S, Vorobiof D, Duarte R, Gil Gil M, Bodrogi I, Murray E, et al. 303 Study Group. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1999;17:2341–2354. - PubMed
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

