Discontinuation of antidepressant medication after mindfulness-based cognitive therapy for recurrent depression: randomised controlled non-inferiority trial
- PMID: 26892847
- PMCID: PMC4816973
- DOI: 10.1192/bjp.bp.115.168971
Discontinuation of antidepressant medication after mindfulness-based cognitive therapy for recurrent depression: randomised controlled non-inferiority trial
Abstract
Background: Mindfulness-based cognitive therapy (MBCT) and maintenance antidepressant medication (mADM) both reduce the risk of relapse in recurrent depression, but their combination has not been studied.
Aims: To investigate whether MBCT with discontinuation of mADM is non-inferior to MBCT+mADM.
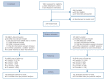
Method: A multicentre randomised controlled non-inferiority trial (ClinicalTrials.gov:NCT00928980). Adults with recurrent depression in remission, using mADM for 6 months or longer (n= 249), were randomly allocated to either discontinue (n= 128) or continue (n= 121) mADM after MBCT. The primary outcome was depressive relapse/recurrence within 15 months. A confidence interval approach with a margin of 25% was used to test non-inferiority. Key secondary outcomes were time to relapse/recurrence and depression severity.
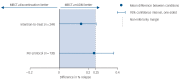
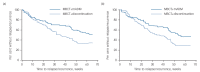
Results: The difference in relapse/recurrence rates exceeded the non-inferiority margin and time to relapse/recurrence was significantly shorter after discontinuation of mADM. There were only minor differences in depression severity.
Conclusions: Our findings suggest an increased risk of relapse/recurrence in patients withdrawing from mADM after MBCT.
© The Royal College of Psychiatrists 2016.
Conflict of interest statement
None.
Figures

References
-
- Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev 2011; 31: 1117–25. - PubMed
-
- Kaymaz N, van Os J, Loonen AJ, Nolen WA. Evidence that patients with single versus recurrent depressive episodes are differentially sensitive to treatment discontinuation: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry 2008; 69: 1423–36. - PubMed
-
- Borges S, Chen YF, Laughren TP, Temple R, Patel HD, David PA, et al. Review of maintenance trials for major depressive disorder: a 25-year perspective from the US Food and Drug Administration. J Clin Psychiatry 2014; 75: 205–14. - PubMed
-
- Viguera AC, Baldessarini RJ, Friedberg J. Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry 1998; 5: 293–306. - PubMed
-
- American Psychiatric Association Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd edn. APA, 2010.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

