MicroRNA Regulation of Atherosclerosis
- PMID: 26892968
- PMCID: PMC4762069
- DOI: 10.1161/CIRCRESAHA.115.306300
MicroRNA Regulation of Atherosclerosis
Abstract
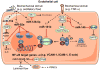
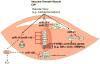
Atherosclerosis and its attendant clinical complications, such as myocardial infarction, stroke, and peripheral artery disease, are the leading cause of morbidity and mortality in Western societies. In response to biochemical and biomechanical stimuli, atherosclerotic lesion formation occurs from the participation of a range of cell types, inflammatory mediators, and shear stress. Over the past decade, microRNAs (miRNAs) have emerged as evolutionarily conserved, noncoding small RNAs that serve as important regulators and fine-tuners of a range of pathophysiological cellular effects and molecular signaling pathways involved in atherosclerosis. Accumulating studies reveal the importance of miRNAs in regulating key signaling and lipid homeostasis pathways that alter the balance of atherosclerotic plaque progression and regression. In this review, we highlight current paradigms of miRNA-mediated effects in atherosclerosis progression and regression. We provide an update on the potential use of miRNAs diagnostically for detecting increasing severity of coronary disease and clinical events. Finally, we provide a perspective on therapeutic opportunities and challenges for miRNA delivery in the field.
Keywords: atherosclerosis; coronary artery disease; lipoproteins; microRNAs; vascular cell adhesion molecule.
© 2016 American Heart Association, Inc.
Figures

References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. - PubMed
-
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

