Imaging Atherosclerosis
- PMID: 26892971
- PMCID: PMC4756468
- DOI: 10.1161/CIRCRESAHA.115.306247
Imaging Atherosclerosis
Abstract
Advances in atherosclerosis imaging technology and research have provided a range of diagnostic tools to characterize high-risk plaque in vivo; however, these important vascular imaging methods additionally promise great scientific and translational applications beyond this quest. When combined with conventional anatomic- and hemodynamic-based assessments of disease severity, cross-sectional multimodal imaging incorporating molecular probes and other novel noninvasive techniques can add detailed interrogation of plaque composition, activity, and overall disease burden. In the catheterization laboratory, intravascular imaging provides unparalleled access to the world beneath the plaque surface, allowing tissue characterization and measurement of cap thickness with micrometer spatial resolution. Atherosclerosis imaging captures key data that reveal snapshots into underlying biology, which can test our understanding of fundamental research questions and shape our approach toward patient management. Imaging can also be used to quantify response to therapeutic interventions and ultimately help predict cardiovascular risk. Although there are undeniable barriers to clinical translation, many of these hold-ups might soon be surpassed by rapidly evolving innovations to improve image acquisition, coregistration, motion correction, and reduce radiation exposure. This article provides a comprehensive review of current and experimental atherosclerosis imaging methods and their uses in research and potential for translation to the clinic.
Keywords: atherosclerosis; coronary artery disease; molecular imaging; multimodal imaging; risk factors.
© 2016 The Authors.
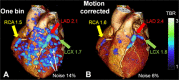
Figures



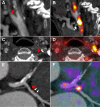
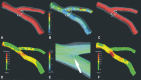
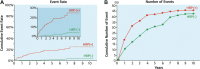
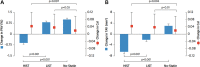
References
-
- Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. - PubMed
-
- Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. - PMC - PubMed
-
- Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

