An external sensing system in Plasmodium falciparum-infected erythrocytes
- PMID: 26893139
- PMCID: PMC4759932
- DOI: 10.1186/s12936-016-1144-6
An external sensing system in Plasmodium falciparum-infected erythrocytes
Abstract
Background: A number of experiments have previously indicated that Plasmodium falciparum-infected erythrocytes (pRBC) were able to sense host environment. The basis of this ability to detect external cues is not known but in screening signalling molecules from pRBC using commercial antibodies, a 34 kDa phosphorylated molecule that possesses such ability was identified.
Methods: The pRBC were exposed to different culture conditions and proteins were extracted for 1D or 2D gel electrophoresis followed by Western blot. The localization of 34 kDa protein was examined by biochemical fractionation followed by Western blot. High-resolution mass spectrometric analysis of immune precipitants was used to identify this protein and real-time quantitative reverse transcriptase polymerase chain reaction was used for detecting mRNA expression level.
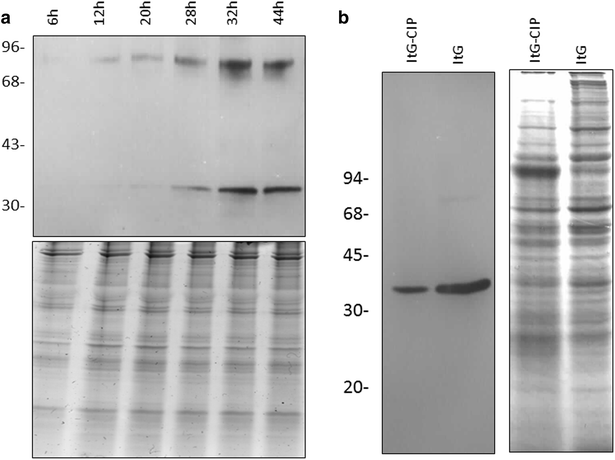
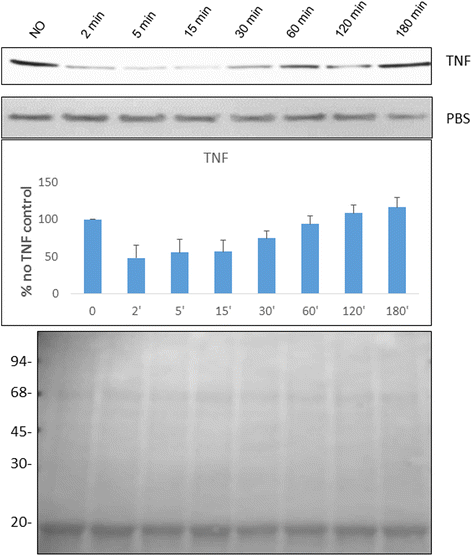
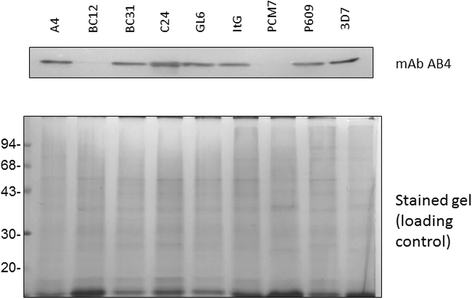
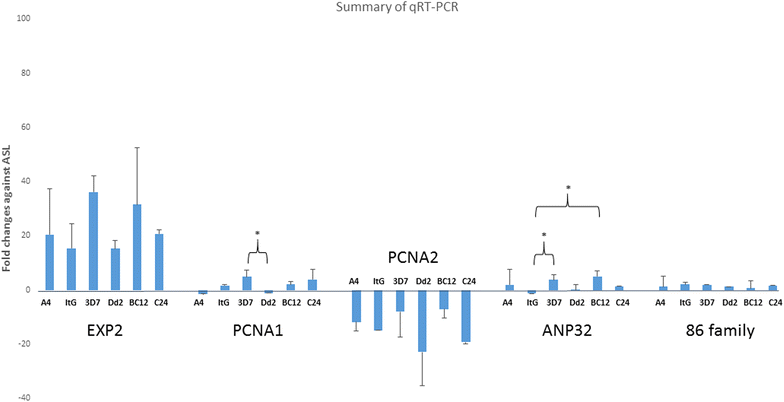
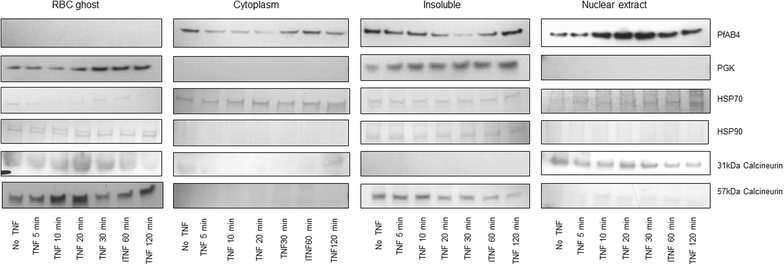
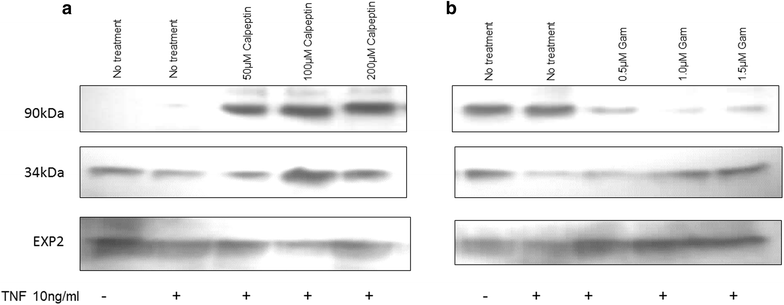
Results: The 34 kDa protein was called PfAB4 has immediate responses (dephosphorylation and rapid turnover) to host environmental stimuli such as serum depletion, osmolality change and cytokine addition. PfAB4 is expressed constitutively throughout the erythrocytic lifecycle with dominant expression in trophozoites 30 h post-infection. Tumour necrosis factor (TNF) treatment induced a transient detectable dephosphorylation of PfAB4 in the ItG strain (2 min after addition) and the level of expression and phosphorylation returned to normal within 1-2 h. PfAB4 localized dominantly in pRBC cytoplasm, with a transient shift to the nucleus under TNF stimulation as shown by biochemical fractionation. High-resolution mass spectrometric analysis of immune precipitants of AB4 antibodies revealed a 34 kDa PfAB4 component as a mixture of proliferating cellular nuclear antigen-1 (PCNA1) and exported protein-2 (EXP2), along with a small number of other inconsistently identified peptides. Different parasite strains have different PfAB4 expression levels, but no significant association between mRNA and PfAB4 levels was seen, indicating that the differences may be at the post-transcriptional, presumably phosphorylation, level. A triple serine phosphorylated PCNA1 peptide was identified from the PfAB4 high expression strain only, providing further evidence that the identity of PfAB4 is PCNA1 in P. falciparum.
Conclusion: A protein element in the human malaria parasite that responds to external cues, including the pro-inflammatory cytokine TNF have been discovered. Treatment results in a transient change in phosphorylation status of the response element, which also migrates from the parasite cytoplasm to the nucleus. The response element has been identified as PfPCNA1. This sensing response could be regulated by a parasite checkpoint system and be analogous to bacterial two-component signal transduction systems.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

