Intravenous levetiracetam versus phenobarbital in children with status epilepticus or acute repetitive seizures
- PMID: 26893602
- PMCID: PMC4753198
- DOI: 10.3345/kjp.2016.59.1.35
Intravenous levetiracetam versus phenobarbital in children with status epilepticus or acute repetitive seizures
Abstract
Purpose: This study compared the efficacy and tolerability of intravenous (i.v.) phenobarbital (PHB) and i.v. levetiracetam (LEV) in children with status epilepticus (SE) or acute repetitive seizure (ARS).
Methods: The medical records of children (age range, 1 month to 15 years) treated with i.v. PHB or LEV for SE or ARS at our single tertiary center were retrospectively reviewed. Seizure termination was defined as seizure cessation within 30 minutes of infusion completion and no recurrence within 24 hours. Information on the demographic variables, electroencephalography and magnetic resonance imaging findings, previous antiepileptic medications, and adverse events after drug infusion was obtained.
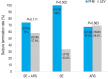
Results: The records of 88 patients with SE or ARS (median age, 18 months; 50 treated with PHB and 38 with LEV) were reviewed. The median initial dose of i.v. PHB was 20 mg/kg (range, 10-20 mg/kg) and that of i.v. LEV was 30 mg/kg (range, 20-30 mg/kg). Seizure termination occurred in 57.9% of patients treated with i.v. LEV (22 of 38) and 74.0% treated with i.v. PHB (37 of 50) (P=0.111). The factor associated with seizure termination was the type of event (SE vs. ARS) in each group. Adverse effects were reported in 13.2% of patients treated with i.v. LEV (5 of 38; n=4, aggressive behavior and n=1, vomiting), and 28.0% of patients treated with i.v. PHB (14 of 50).
Conclusion: Intravenous LEV was efficacious and safe in children with ARS or SE. Further evaluation is needed to determine the most effective and best-tolerated loading dose of i.v. LEV.
Keywords: Child; Levetiracetam; Phenobarbital; Seizures; Status epilepticus.
Conflict of interest statement
Figures
References
-
- Silbergleit R National Institutes of Health/National Institute of Neurological Diseases and Stroke Neurological Emergencies Treatment Trials Investigators. Response to Food and Drug Administration draft guidance statement on research into the treatment of life-threatening emergency conditions using exception from informed consent: testimony of the neurological emergencies treatment trials. Acad Emerg Med. 2007;14:e63–e68. - PubMed
-
- Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–798. - PubMed
-
- Appleton R, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol. 1995;37:682–688. - PubMed
-
- Eue S, Grumbt M, Muller M, Schulze A. Two years of experience in the treatment of status epilepticus with intravenous levetiracetam. Epilepsy Behav. 2009;15:467–469. - PubMed
-
- Aiguabella M, Falip M, Villanueva V, de la Pena P, Molins A, Garcia-Morales I, et al. Efficacy of intravenous levetiracetam as an add-on treatment in status epilepticus: a multicentric observational study. Seizure. 2011;20:60–64. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources