Antacid Use and De Novo Brain Metastases in Patients with Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Who Were Treated Using First-Line First-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors
- PMID: 26894507
- PMCID: PMC4760710
- DOI: 10.1371/journal.pone.0149722
Antacid Use and De Novo Brain Metastases in Patients with Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Who Were Treated Using First-Line First-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors
Abstract
Background: Antacid treatments decrease the serum concentrations of first-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs), although it is unknown whether antacids affect clinical outcomes. As cerebrospinal fluid concentrations of TKIs are much lower than serum concentrations, we hypothesized that this drug-drug interaction might affect the prognosis of patients with de novo brain metastases.
Materials and methods: This retrospective study evaluated 269 patients with EGFR-mutant non-small cell lung cancer (NSCLC) who had been diagnosed between December 2010 and December 2013, and had been treated using first-line first-generation EGFR-TKIs. Among these patients, we identified patients who concurrently used H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) as antacids. Patients who exhibited >30% overlap between the use of TKIs and antacids were considered antacid users.
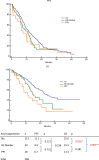
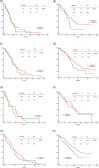
Results: Fifty-seven patients (57/269, 21.2%) were antacid users, and antacid use did not significantly affect progression-free survival (PFS; no antacids: 11.2 months, H2RAs: 9.4 months, PPIs: 6.7 months; p = 0.234). However, antacid use significantly reduced overall survival (OS; no antacids: 25.0 months, H2RAs: 15.5 months, PPIs: 11.3 months; p = 0.002). Antacid use did not affect PFS for various metastasis sites, although antacid users with de novo brain metastases exhibited significantly shorter OS, compared to non-users (11.8 vs. 16.3 months, respectively; p = 0.041). Antacid use did not significantly affect OS in patients with bone, liver, or pleural metastases.
Conclusion: Antacid use reduced OS among patients with EGFR-mutant NSCLC who were treated using first-line first-generation EGFR-TKIs, and especially among patients with de novo brain metastases.
Conflict of interest statement
Figures

Similar articles
-
Effects of epidermal growth factor receptor-tyrosine kinase inhibitors alone on EGFR-mutant non-small cell lung cancer with brain metastasis.Thorac Cancer. 2016 Nov;7(6):648-654. doi: 10.1111/1759-7714.12379. Epub 2016 Sep 1. Thorac Cancer. 2016. PMID: 27755835 Free PMC article.
-
Baseline and Trend of Lymphocyte-to-Monocyte Ratio as Prognostic Factors in Epidermal Growth Factor Receptor Mutant Non-Small Cell Lung Cancer Patients Treated with First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors.PLoS One. 2015 Aug 27;10(8):e0136252. doi: 10.1371/journal.pone.0136252. eCollection 2015. PLoS One. 2015. PMID: 26313661 Free PMC article.
-
Is there any predictor for clinical outcome in EGFR mutant NSCLC patients treated with EGFR TKIs?Cancer Chemother Pharmacol. 2014 May;73(5):1063-70. doi: 10.1007/s00280-014-2442-8. Epub 2014 Mar 25. Cancer Chemother Pharmacol. 2014. PMID: 24663503
-
Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor -mutant non-small cell lung cancer.Lung Cancer. 2016 Mar;93:59-68. doi: 10.1016/j.lungcan.2016.01.003. Epub 2016 Jan 8. Lung Cancer. 2016. PMID: 26898616 Review.
-
Brain Metastases in NSCLC - are TKIs Changing the Treatment Strategy?Anticancer Res. 2015 Nov;35(11):5797-806. Anticancer Res. 2015. PMID: 26504000 Review.
Cited by
-
Evaluation of Drug-Drug Interactions in EGFR-Mutated Non-Small-Cell Lung Cancer Patients during Treatment with Tyrosine-Kinase Inhibitors.J Pers Med. 2021 May 18;11(5):424. doi: 10.3390/jpm11050424. J Pers Med. 2021. PMID: 34069851 Free PMC article.
-
Proton pump inhibitors reduce the survival of advanced lung cancer patients with therapy of gefitinib or erlotinib.Sci Rep. 2022 Apr 29;12(1):7002. doi: 10.1038/s41598-022-10938-x. Sci Rep. 2022. PMID: 35488047 Free PMC article.
-
Alkalescent soda beverage caused the disappearance of gefitinib-induced rashes and decreased efficacy in a non-small-cell lung cancer patient treated with gefitinib: A case report.Respir Med Case Rep. 2020 Sep 16;31:101228. doi: 10.1016/j.rmcr.2020.101228. eCollection 2020. Respir Med Case Rep. 2020. PMID: 32995264 Free PMC article.
-
A Survival Scoring System for Non-Small Cell Lung Cancer Patients with De Novo Bone Metastases.PLoS One. 2016 Dec 8;11(12):e0167923. doi: 10.1371/journal.pone.0167923. eCollection 2016. PLoS One. 2016. PMID: 27930702 Free PMC article.
-
The impact of de novo liver metastasis on clinical outcome in patients with advanced non-small-cell lung cancer.PLoS One. 2017 Jun 7;12(6):e0178676. doi: 10.1371/journal.pone.0178676. eCollection 2017. PLoS One. 2017. PMID: 28591157 Free PMC article.
References
-
- Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in taiwan: a population-based cancer registry. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8(9):1128–35. 10.1097/JTO.0b013e31829ceba4 . - DOI - PubMed
-
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13306–11. 10.1073/pnas.0405220101 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

