Drug-Drug Interactions Between the Anti-Hepatitis C Virus 3D Regimen of Ombitasvir, Paritaprevir/Ritonavir, and Dasabuvir and Eight Commonly Used Medications in Healthy Volunteers
- PMID: 26895022
- PMCID: PMC4933729
- DOI: 10.1007/s40262-016-0373-8
Drug-Drug Interactions Between the Anti-Hepatitis C Virus 3D Regimen of Ombitasvir, Paritaprevir/Ritonavir, and Dasabuvir and Eight Commonly Used Medications in Healthy Volunteers
Abstract
Background and aims: The three direct-acting antiviral regimen of ombitasvir/paritaprevir/ritonavir and dasabuvir (3D regimen) is approved for treatment of hepatitis C virus (HCV) genotype 1 infection. Drug-drug interaction (DDI) studies of the 3D regimen and commonly used medications were conducted in healthy volunteers to provide information on coadministering these medications with or without dose adjustments.
Methods: Three phase I studies evaluated DDIs between the 3D regimen (ombitasvir/paritaprevir/ritonavir 25/150/100 mg once daily + dasabuvir 250 mg twice daily) and hydrocodone bitartrate/acetaminophen (5/300 mg), metformin hydrochloride (500 mg), diazepam (2 mg), cyclobenzaprine hydrochloride (5 mg), carisoprodol (250 mg), or sulfamethoxazole/trimethoprim (SMZ/TMP) (800/160 mg twice daily), all administered orally. DDI magnitude was determined using geometric mean ratios and 90 % confidence intervals for the maximum plasma concentration (C max) and area under the plasma concentration-time curve (AUC).
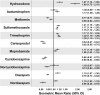
Results: Changes in exposures (C max and AUC geometric mean ratios) of acetaminophen, metformin, sulfamethoxazole, trimethoprim, and diazepam were ≤25 % upon coadministration with the 3D regimen. The C max and AUC of nordiazepam, an active metabolite of diazepam, increased by 10 % and decreased by 44 %, respectively. Exposures of cyclobenzaprine and carisoprodol decreased by ≤40 and ≤46 %, respectively, whereas exposures of hydrocodone increased up to 90 %. Ombitasvir, paritaprevir, ritonavir, and dasabuvir exposures changed by ≤25 %, except for a 37 % decrease in paritaprevir C max with metformin and a 33 % increase in dasabuvir AUC with SMZ/TMP.
Conclusions: Acetaminophen, metformin, sulfamethoxazole, and trimethoprim can be coadministered with the 3D regimen without dose adjustment. Higher doses may be needed for diazepam, cyclobenzaprine, and carisoprodol based on clinical monitoring. A 50 % lower dose and/or clinical monitoring should be considered for hydrocodone. No dose adjustment is necessary for the 3D regimen.
Figures


References
-
- Patel N, Nasiri M, Koroglu A, Bliss S, Davis M, McNutt LA, et al. A cross-sectional study comparing the frequency of drug interactions after adding simeprevir- or sofosbuvir-containing therapy to medication profiles of hepatitis C monoinfected patients. Infect Dis Ther. 2015;4:67–78. doi: 10.1007/s40121-015-0058-x. - DOI - PMC - PubMed
-
- Lauffenburger JC, Mayer CL, Hawke RL, Brouwer KL, Fried MW, Farley JF. Medication use and medical comorbidity in patients with chronic hepatitis C from a US commercial claims database: high utilization of drugs with interaction potential. Eur J Gastroenterol Hepatol. 2014;26:1073–1082. doi: 10.1097/MEG.0000000000000152. - DOI - PMC - PubMed
-
- Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97 and 100 % sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365. doi: 10.1053/j.gastro.2014.04.045. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

