LEAP: L1 Element Amplification Protocol
- PMID: 26895063
- PMCID: PMC5070798
- DOI: 10.1007/978-1-4939-3372-3_21
LEAP: L1 Element Amplification Protocol
Abstract
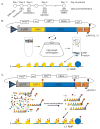
Long INterspersed Element-1 (LINE-1 or L1) retrotransposons encode two proteins (ORF1p and ORF2p) that are required for retrotransposition. The L1 element amplification protocol (LEAP) assays the ability of L1 ORF2p to reverse transcribe L1 RNA in vitro. Ultracentrifugation or immunoprecipitation is used to isolate L1 ribonucleoprotein particle (RNP) complexes from cultured human cells transfected with an engineered L1 expression construct. The isolated RNPs are incubated with an oligonucleotide that contains a unique sequence at its 5' end and a thymidine-rich sequence at its 3' end. The addition of dNTPs to the reaction allows L1 ORF2p bound to L1 RNA to generate L1 cDNA. The resultant L1 cDNAs then are amplified using polymerase chain reaction (PCR) and the products are visualized by gel electrophoresis. Sequencing the resultant PCR products then allows product verification. The LEAP assay has been instrumental in determining how mutations in L1 ORF1p and ORF2p affect L1 reverse transcriptase (RT) activity. Furthermore, the LEAP assay has revealed that the L1 ORF2p RT can extend a DNA primer with mismatched 3' terminal bases when it is annealed to an L1 RNA template. As the LINE-1 biology field gravitates toward studying cellular proteins that regulate LINE-1, molecular genetic and biochemical approaches such as LEAP, in conjunction with the LINE-1-cultured cell retrotransposition assay, are essential to dissect the molecular mechanism of L1 retrotransposition.
Keywords: L1 element amplification protocol (LEAP); LINE-1; Reverse transcriptase (RT); Ribonucleoprotein particle (RNP).
Conflict of interest statement
J.V.M. is an inventor on the patent: “Kazazian, H.H., Boeke, J.D., Moran, J.V., and Dombrowski, B.A. Compositions and methods of use of mammalian retrotransposons. Application No. 60/006,831; Patent No. 6,150,160; Issued November 21, 2000.” J.V.M. has not made any money from this patent and voluntarily discloses this information.
Figures


References
-
- Dombroski BA, Mathias SL, Nanthakumar E, Scott AF, Kazazian HH., Jr Isolation of an active human transposable element. Science. 1991;254:1805–1808. - PubMed
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
-
- Sassaman DM, Dombroski BA, Moran JV, Kimberland ML, Naas TP, DeBerardinis RJ, Gabriel A, Swergold GD, Kazazian HH., Jr Many human L1 elements are capable of retrotransposition. Nat Genet. 1997;16:37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

